中国全科医学 ›› 2026, Vol. 29 ›› Issue (06): 710-717.DOI: 10.12114/j.issn.1007-9572.2025.0208
杨海飞1,2, 孙武2, 吴成2, 任伟2, 李茹恬1,2,*( )
)
收稿日期:2025-06-15
修回日期:2025-07-18
出版日期:2026-02-20
发布日期:2026-01-05
通讯作者:
李茹恬
作者贡献:
杨海飞负责论文撰写与数据收集;孙武进行数据的收集与整理;吴成负责统计学处理,图、表的绘制与展示;任伟进行论文的修订,负责文章的质量控制与审查;李茹恬提出主要研究目标,负责研究的构思与设计、监督管理,对文章整体负责。
基金资助:
YANG Haifei1,2, SUN Wu2, WU Cheng2, REN Wei2, LI Rutian1,2,*( )
)
Received:2025-06-15
Revised:2025-07-18
Published:2026-02-20
Online:2026-01-05
Contact:
LI Rutian
摘要: 背景 免疫治疗已成为进展期食管鳞状细胞癌(ESCC)的标准治疗方案,但目前尚缺乏明确的预测免疫相关不良反应(irAEs)的生物标志物,且irAEs与疗效的关系亦不明确。 目的 探讨进展期ESCC患者发生irAEs的预测因素及irAEs与疗效的相关性。 方法 回顾性纳入2020—2023年在南京鼓楼医院接受程序性死亡蛋白1(PD-1)抑制剂治疗的118例进展期ESCC患者为研究对象,通过查阅病历、门诊、再入院、电话等方式对入组患者进行随访,收集患者的临床资料及irAEs情况,根据治疗过程中是否发生irAEs分为irAEs阳性组和irAEs阴性组,采用单因素和多因素Logistic回归分析探讨影响irAEs发生的相关因素。疗效评价为完全缓解(CR)、部分缓解(PR)、疾病稳定(SD)、疾病进展(PD),比较两组的客观缓解率(ORR)、疾病控制率(DCR),采用Kaplan-Meier法绘制生存曲线,采用Log-rank检验比较两组患者无进展生存期(PFS)及总生存期(OS)差异。 结果 118例患者中47例(39.83%)发生irAEs,发生率较高的irAEs为皮肤毒性21例(17.80%)、内分泌毒性16例(13.56%)和肺毒性16例(13.56%)。irAEs阳性组和irAEs阴性组自身抗体谱及抗核抗体(ANA)比较,差异有统计学意义(P<0.05)。单因素Logistic分析显示,自身抗体谱阳性(OR=3.375,95%CI=1.527~7.456,P=0.003)和ANA阳性(OR=3.072,95%CI=1.404~6.722,P=0.005)是irAEs发生的危险因素(P<0.05);多因素Logistic分析结果显示,自身抗体谱阳性(OR=2.367,95%CI=0.841~6.663,P=0.103)和ANA阳性(OR=1.733,95%CI=0.621~4.837,P=0.293)与irAEs的发生无显著关联;但自身抗体谱阳性患者内分泌毒性的发生率高于自身抗体谱阴性患者,ANA阳性患者内分泌毒性和肌毒性的发生率高于ANA阴性患者(P<0.05)。且irAEs阳性组DCR高于irAEs阴性组(χ2=6.690,P=0.010);两组ORR比较,差异无统计学意义(χ2=2.628,P=0.105)。生存分析结果显示,irAEs阳性组中位PFS和中位OS长于irAEs阴性组,差异均有统计学意义(PFS:χ2=9.521,P=0.002;OS:χ2=4.254,P=0.039)。 结论 自身抗体谱或ANA阳性的ESCC患者接受免疫治疗后可能更容易发生irAEs,且irAEs的发生与更好的治疗疗效相关。
中图分类号:
| 组别 | 例数 | 年龄(岁) | 性别[例(%)] | TNM分期[例(%)] | ECOG评分[例(%)] | CPS[例(%)] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 男 | 女 | Ⅲ期 | Ⅳ期 | 0~1分 | ≥2分 | 阴性 | 阳性 | NA | |||
| irAEs阴性组 | 71 | 68.0 ± 8.1 | 60(84.51) | 11(15.49) | 16(22.54) | 55(77.46) | 65(91.55) | 6(8.45) | 20(28.17) | 8(11.27) | 43(60.56) |
| irAEs阳性组 | 47 | 66.8 ± 8.1 | 37(78.72) | 10(21.28) | 10(21.28) | 37(78.72) | 43(91.49) | 4(8.51) | 19(40.43) | 10(21.28) | 18(38.30) |
| χ2(t)值 | 0.828a | 0.647 | 0.026 | <0.001 | 5.855 | ||||||
| P值 | 0.409 | 0.421 | 0.872 | 1.000 | 0.054 | ||||||
| 组别 | 肿瘤分化[例(%)] | 肿瘤位置[例(%)] | 肝转移[例(%)] | ||||||||
| 高分化 | 中分化 | 低分化 | 胸上段 | 胸中段 | 胸下段 | 阴性 | 阳性 | ||||
| irAEs阴性组 | 14(19.72) | 43(60.56) | 14(19.72) | 16(22.54) | 32(45.07) | 23(32.39) | 63(88.73) | 8(11.27) | |||
| irAEs阳性组 | 10(21.28) | 24(51.06) | 13(27.66) | 15(31.91) | 19(40.43) | 13(27.66) | 43(91.49) | 4(8.51) | |||
| χ2(t)值 | 1.263 | 1.296 | 0.030 | ||||||||
| P值 | 0.532 | 0.523 | 0.862 | ||||||||
| 组别 | 肺转移[例(%)] | 复发部位数量[例(%)] | 自身抗体谱[例(%)] | ANA[例(%)] | |||||||
| 阴性 | 阳性 | 1个 | ≥2个 | 阴性 | 阳性 | 阴性 | 阳性 | ||||
| irAEs阴性组 | 66(92.96) | 5(7.04) | 60(84.51) | 11(15.49) | 40(56.34) | 31(43.66) | 53(74.65) | 18(25.35) | |||
| irAEs阳性组 | 41(87.23) | 6(12.77) | 38(80.85) | 9(19.15) | 13(27.66) | 34(72.34) | 23(48.94) | 24(51.06) | |||
| χ2(t)值 | 0.523 | 0.269 | 9.401 | 8.155 | |||||||
| P值 | 0.469 | 0.604 | 0.002 | 0.004 | |||||||
| 组别 | 抗Ro-52抗体[例(%)] | 治疗线数[例(%)] | PD-1抑制剂[例(%)] | ||||||||
| 阴性 | 阳性 | 一线 | 二线及以上 | 卡瑞利珠单抗 | 特瑞普利单抗 | 信迪利单抗 | 替雷利珠单抗 | ||||
| irAEs阴性组 | 61(85.92) | 10(14.08) | 69(97.18) | 2(2.82) | 30(42.25) | 9(12.68) | 20(28.17) | 12(16.90) | |||
| irAEs阳性组 | 41(87.23) | 6(12.77) | 45(95.74) | 2(4.26) | 16(34.04) | 9(19.15) | 12(25.53) | 10(21.28) | |||
| χ2(t)值 | 0.042 | <0.001 | 1.629 | ||||||||
| P值 | 0.838 | 1.000 | 0.653 | ||||||||
表1 irAEs阳性组和irAEs阴性组一般资料比较
Table 1 Comparison of general data between the irAEs-positive group and the irAEs-negative groups
| 组别 | 例数 | 年龄(岁) | 性别[例(%)] | TNM分期[例(%)] | ECOG评分[例(%)] | CPS[例(%)] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 男 | 女 | Ⅲ期 | Ⅳ期 | 0~1分 | ≥2分 | 阴性 | 阳性 | NA | |||
| irAEs阴性组 | 71 | 68.0 ± 8.1 | 60(84.51) | 11(15.49) | 16(22.54) | 55(77.46) | 65(91.55) | 6(8.45) | 20(28.17) | 8(11.27) | 43(60.56) |
| irAEs阳性组 | 47 | 66.8 ± 8.1 | 37(78.72) | 10(21.28) | 10(21.28) | 37(78.72) | 43(91.49) | 4(8.51) | 19(40.43) | 10(21.28) | 18(38.30) |
| χ2(t)值 | 0.828a | 0.647 | 0.026 | <0.001 | 5.855 | ||||||
| P值 | 0.409 | 0.421 | 0.872 | 1.000 | 0.054 | ||||||
| 组别 | 肿瘤分化[例(%)] | 肿瘤位置[例(%)] | 肝转移[例(%)] | ||||||||
| 高分化 | 中分化 | 低分化 | 胸上段 | 胸中段 | 胸下段 | 阴性 | 阳性 | ||||
| irAEs阴性组 | 14(19.72) | 43(60.56) | 14(19.72) | 16(22.54) | 32(45.07) | 23(32.39) | 63(88.73) | 8(11.27) | |||
| irAEs阳性组 | 10(21.28) | 24(51.06) | 13(27.66) | 15(31.91) | 19(40.43) | 13(27.66) | 43(91.49) | 4(8.51) | |||
| χ2(t)值 | 1.263 | 1.296 | 0.030 | ||||||||
| P值 | 0.532 | 0.523 | 0.862 | ||||||||
| 组别 | 肺转移[例(%)] | 复发部位数量[例(%)] | 自身抗体谱[例(%)] | ANA[例(%)] | |||||||
| 阴性 | 阳性 | 1个 | ≥2个 | 阴性 | 阳性 | 阴性 | 阳性 | ||||
| irAEs阴性组 | 66(92.96) | 5(7.04) | 60(84.51) | 11(15.49) | 40(56.34) | 31(43.66) | 53(74.65) | 18(25.35) | |||
| irAEs阳性组 | 41(87.23) | 6(12.77) | 38(80.85) | 9(19.15) | 13(27.66) | 34(72.34) | 23(48.94) | 24(51.06) | |||
| χ2(t)值 | 0.523 | 0.269 | 9.401 | 8.155 | |||||||
| P值 | 0.469 | 0.604 | 0.002 | 0.004 | |||||||
| 组别 | 抗Ro-52抗体[例(%)] | 治疗线数[例(%)] | PD-1抑制剂[例(%)] | ||||||||
| 阴性 | 阳性 | 一线 | 二线及以上 | 卡瑞利珠单抗 | 特瑞普利单抗 | 信迪利单抗 | 替雷利珠单抗 | ||||
| irAEs阴性组 | 61(85.92) | 10(14.08) | 69(97.18) | 2(2.82) | 30(42.25) | 9(12.68) | 20(28.17) | 12(16.90) | |||
| irAEs阳性组 | 41(87.23) | 6(12.77) | 45(95.74) | 2(4.26) | 16(34.04) | 9(19.15) | 12(25.53) | 10(21.28) | |||
| χ2(t)值 | 0.042 | <0.001 | 1.629 | ||||||||
| P值 | 0.838 | 1.000 | 0.653 | ||||||||
| 变量 | β | SE | Z值 | P值 | OR(95%CI) |
|---|---|---|---|---|---|
| 年龄 | -0.019 | 0.023 | -0.831 | 0.406 | 0.981(0.937~1.027) |
| 性别(以男为参照) | |||||
| 女 | 0.388 | 0.484 | 0.801 | 0.423 | 1.474(0.571~3.809) |
| TNM分期(以Ⅲ期为参照) | |||||
| Ⅳ期 | 0.074 | 0.456 | 0.161 | 0.872 | 1.076(0.441~2.630) |
| ECOG评分(以0~1分为参照) | |||||
| ≥2分 | 0.008 | 0.675 | 0.011 | 0.991 | 1.008(0.269~3.782) |
| CPS(以阴性为参照) | |||||
| 阳性 | 0.274 | 0.572 | 0.479 | 0.632 | 1.316(0.429~4.040) |
| NA | -0.820 | 0.426 | -1.924 | 0.054 | 0.441(0.191~1.015) |
| 肿瘤分化(以高分化为参照) | |||||
| 中分化 | -0.247 | 0.486 | -0.507 | 0.612 | 0.781(0.301~2.026) |
| 低分化 | 0.262 | 0.565 | 0.464 | 0.643 | 1.300(0.429~3.938) |
| 肿瘤位置(以胸上段为参照) | |||||
| 胸中段 | -0.457 | 0.462 | -0.990 | 0.322 | 0.633(0.256~1.565) |
| 胸下段 | -0.506 | 0.500 | -1.013 | 0.311 | 0.603(0.226~1.605) |
| 肝转移(以阴性为参照) | |||||
| 阳性 | -0.311 | 0.644 | -0.484 | 0.629 | 0.733(0.208~2.586) |
| 肺转移(以阴性为参照) | |||||
| 阳性 | 0.658 | 1.033 | 0.637 | 0.302 | 1.932(0.554~6.737) |
| 复发部位数量(以1个为参照) | |||||
| ≥2个 | 0.256 | 0.495 | 0.517 | 0.605 | 1.292(0.490~3.408) |
| 自身抗体谱(以阴性为参照) | |||||
| 阳性 | 1.216 | 0.404 | 3.007 | 0.003 | 3.375(1.527~7.456) |
| ANA(以阴性为参照) | |||||
| 阳性 | 1.122 | 0.399 | 2.810 | 0.005 | 3.072(1.404~6.722) |
| 抗Ro-52抗体(以阴性为参照) | |||||
| 阳性 | -0.114 | 0.554 | -0.205 | 0.838 | 0.893(0.301~2.647) |
| 治疗线数(以一线为参照) | |||||
| 二线及以上 | 0.427 | 1.018 | 0.420 | 0.675 | 1.533(0.208~11.280) |
| PD-1抑制剂(以卡瑞利珠单抗为参照) | |||||
| 特瑞普利单抗 | 0.629 | 0.564 | 1.115 | 0.265 | 1.875(0.621~5.663) |
| 信迪利单抗 | 0.118 | 0.479 | 0.246 | 0.806 | 1.125(0.440~2.875) |
| 替雷利珠单抗 | 0.446 | 0.528 | 0.845 | 0.398 | 1.562(0.555~4.401) |
表2 irAEs发生影响因素的单因素Logistic回归分析
Table 2 Univariate Logistic regression analysis of influencing factors for the occurrence of irAEs
| 变量 | β | SE | Z值 | P值 | OR(95%CI) |
|---|---|---|---|---|---|
| 年龄 | -0.019 | 0.023 | -0.831 | 0.406 | 0.981(0.937~1.027) |
| 性别(以男为参照) | |||||
| 女 | 0.388 | 0.484 | 0.801 | 0.423 | 1.474(0.571~3.809) |
| TNM分期(以Ⅲ期为参照) | |||||
| Ⅳ期 | 0.074 | 0.456 | 0.161 | 0.872 | 1.076(0.441~2.630) |
| ECOG评分(以0~1分为参照) | |||||
| ≥2分 | 0.008 | 0.675 | 0.011 | 0.991 | 1.008(0.269~3.782) |
| CPS(以阴性为参照) | |||||
| 阳性 | 0.274 | 0.572 | 0.479 | 0.632 | 1.316(0.429~4.040) |
| NA | -0.820 | 0.426 | -1.924 | 0.054 | 0.441(0.191~1.015) |
| 肿瘤分化(以高分化为参照) | |||||
| 中分化 | -0.247 | 0.486 | -0.507 | 0.612 | 0.781(0.301~2.026) |
| 低分化 | 0.262 | 0.565 | 0.464 | 0.643 | 1.300(0.429~3.938) |
| 肿瘤位置(以胸上段为参照) | |||||
| 胸中段 | -0.457 | 0.462 | -0.990 | 0.322 | 0.633(0.256~1.565) |
| 胸下段 | -0.506 | 0.500 | -1.013 | 0.311 | 0.603(0.226~1.605) |
| 肝转移(以阴性为参照) | |||||
| 阳性 | -0.311 | 0.644 | -0.484 | 0.629 | 0.733(0.208~2.586) |
| 肺转移(以阴性为参照) | |||||
| 阳性 | 0.658 | 1.033 | 0.637 | 0.302 | 1.932(0.554~6.737) |
| 复发部位数量(以1个为参照) | |||||
| ≥2个 | 0.256 | 0.495 | 0.517 | 0.605 | 1.292(0.490~3.408) |
| 自身抗体谱(以阴性为参照) | |||||
| 阳性 | 1.216 | 0.404 | 3.007 | 0.003 | 3.375(1.527~7.456) |
| ANA(以阴性为参照) | |||||
| 阳性 | 1.122 | 0.399 | 2.810 | 0.005 | 3.072(1.404~6.722) |
| 抗Ro-52抗体(以阴性为参照) | |||||
| 阳性 | -0.114 | 0.554 | -0.205 | 0.838 | 0.893(0.301~2.647) |
| 治疗线数(以一线为参照) | |||||
| 二线及以上 | 0.427 | 1.018 | 0.420 | 0.675 | 1.533(0.208~11.280) |
| PD-1抑制剂(以卡瑞利珠单抗为参照) | |||||
| 特瑞普利单抗 | 0.629 | 0.564 | 1.115 | 0.265 | 1.875(0.621~5.663) |
| 信迪利单抗 | 0.118 | 0.479 | 0.246 | 0.806 | 1.125(0.440~2.875) |
| 替雷利珠单抗 | 0.446 | 0.528 | 0.845 | 0.398 | 1.562(0.555~4.401) |
| 变量 | β | SE | Z值 | P值 | OR(95%CI) |
|---|---|---|---|---|---|
| 自身抗体谱(以阴性为参照) | |||||
| 阳性 | 0.862 | 0.528 | 1.632 | 0.103 | 2.367(0.841~6.663) |
| ANA(以阴性为参照) | |||||
| 阳性 | 0.550 | 0.524 | 1.051 | 0.293 | 1.733(0.621~4.837) |
表3 irAEs发生影响因素的多因素Logistic回归分析
Table 3 Multivariate Logistic regression analysis of influencing factors for the occurrence of irAEs
| 变量 | β | SE | Z值 | P值 | OR(95%CI) |
|---|---|---|---|---|---|
| 自身抗体谱(以阴性为参照) | |||||
| 阳性 | 0.862 | 0.528 | 1.632 | 0.103 | 2.367(0.841~6.663) |
| ANA(以阴性为参照) | |||||
| 阳性 | 0.550 | 0.524 | 1.051 | 0.293 | 1.733(0.621~4.837) |
| irAEs类型 | 自身抗体谱 | ANA | ||||||
|---|---|---|---|---|---|---|---|---|
| 阴性(n=53) | 阳性(n=65) | χ2值 | P值 | 阴性(n=76) | 阳性(n=42) | χ2值 | P值 | |
| 皮肤毒性 | 10(18.87) | 11(16.92) | 0.075 | 0.784 | 14(18.42) | 7(16.67) | 0.057 | 0.811 |
| 内分泌毒性 | 1(1.89) | 15(23.08) | 13.516 | 0.001 | 3(3.95) | 13(30.95) | 16.831 | <0.001 |
| 心脏毒性 | 0 | 3(4.62) | 3.642 | 0.056 | 1(1.32) | 2(4.76) | 1.226 | 0.268 |
| 肺毒性 | 4(7.55) | 12(18.46) | 2.967 | 0.085 | 8(10.53) | 8(19.05) | 1.676 | 0.195 |
| 胃肠道毒性 | 1(1.89) | 2(3.08) | 0.158 | 0.691 | 1(1.32) | 2(4.76) | 1.226 | 0.268 |
| 肝脏毒性 | 2(3.77) | 2(3.08) | 0.043 | 0.836 | 2(2.63) | 2(4.76) | 0.359 | 0.549 |
| 肌毒性 | 0 | 2(3.08) | 2.413 | 0.120 | 0 | 2(4.76) | 4.195 | 0.041 |
| 肾脏毒性 | 0 | 1(1.54) | 1.228 | 0.268 | 1(1.32) | 0 | 0.885 | 0.347 |
表4 不同自身抗体谱和ANA患者irAEs比较[例(%)]
Table 4 Comparisons of irAEs between patients with different autoantibodies and ANA
| irAEs类型 | 自身抗体谱 | ANA | ||||||
|---|---|---|---|---|---|---|---|---|
| 阴性(n=53) | 阳性(n=65) | χ2值 | P值 | 阴性(n=76) | 阳性(n=42) | χ2值 | P值 | |
| 皮肤毒性 | 10(18.87) | 11(16.92) | 0.075 | 0.784 | 14(18.42) | 7(16.67) | 0.057 | 0.811 |
| 内分泌毒性 | 1(1.89) | 15(23.08) | 13.516 | 0.001 | 3(3.95) | 13(30.95) | 16.831 | <0.001 |
| 心脏毒性 | 0 | 3(4.62) | 3.642 | 0.056 | 1(1.32) | 2(4.76) | 1.226 | 0.268 |
| 肺毒性 | 4(7.55) | 12(18.46) | 2.967 | 0.085 | 8(10.53) | 8(19.05) | 1.676 | 0.195 |
| 胃肠道毒性 | 1(1.89) | 2(3.08) | 0.158 | 0.691 | 1(1.32) | 2(4.76) | 1.226 | 0.268 |
| 肝脏毒性 | 2(3.77) | 2(3.08) | 0.043 | 0.836 | 2(2.63) | 2(4.76) | 0.359 | 0.549 |
| 肌毒性 | 0 | 2(3.08) | 2.413 | 0.120 | 0 | 2(4.76) | 4.195 | 0.041 |
| 肾脏毒性 | 0 | 1(1.54) | 1.228 | 0.268 | 1(1.32) | 0 | 0.885 | 0.347 |
| 分组 | 例数 | CR+PR[例(%)] | SD[例(%)] | PD[例(%)] | ORR(%) | DCR(%) |
|---|---|---|---|---|---|---|
| irAEs阴性组 | 71 | 33(46.48) | 27(38.03) | 11(15.49) | 46.48 | 84.51 |
| irAEs阳性组 | 47 | 29(61.70) | 17(36.17) | 1(2.13) | 61.70 | 97.87 |
表5 irAEs阴性组与irAEs阳性组治疗疗效情况
Table 5 Comparison of therapeutic efficacy between the irAEs-negative group and the irAEs-positive group
| 分组 | 例数 | CR+PR[例(%)] | SD[例(%)] | PD[例(%)] | ORR(%) | DCR(%) |
|---|---|---|---|---|---|---|
| irAEs阴性组 | 71 | 33(46.48) | 27(38.03) | 11(15.49) | 46.48 | 84.51 |
| irAEs阳性组 | 47 | 29(61.70) | 17(36.17) | 1(2.13) | 61.70 | 97.87 |
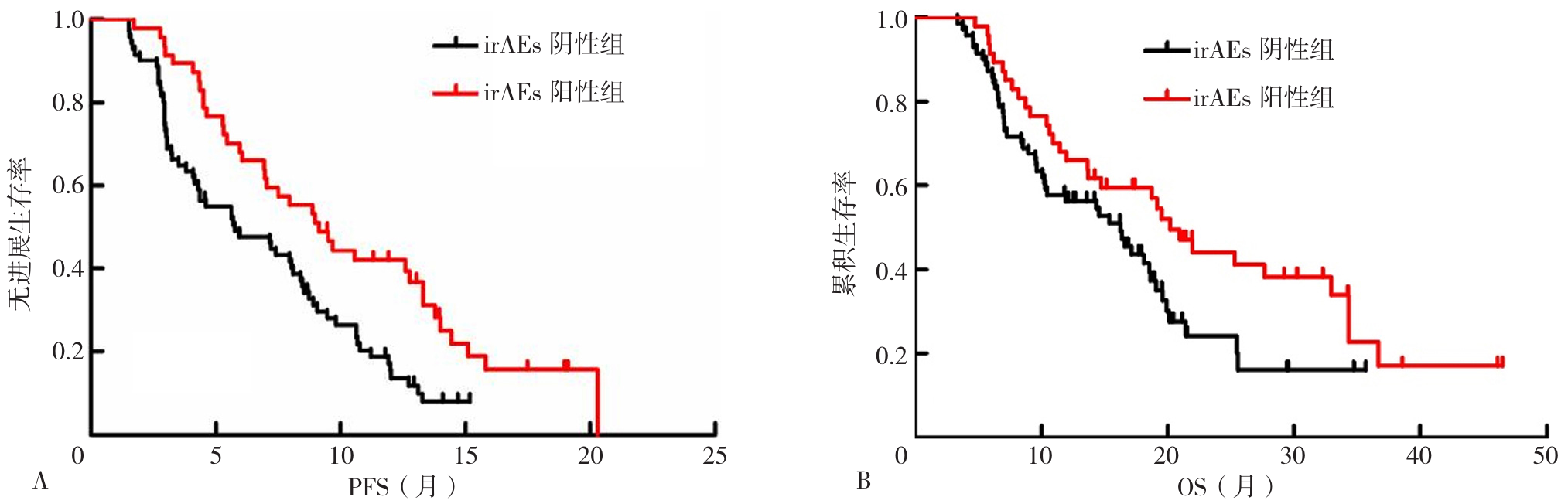
图1 irAEs阳性组和irAEs阴性组患者的Kaplan-Meier生存曲线注:irAEs=免疫相关不良反应,PFS=无进展生存期,OS=总生存期;A为irAEs阴性组和irAEs的阳性组PFS生存曲线,B为irAEs阴性组和irAEs阳性组的OS生存曲线。
Figure 1 Kaplan-Meier survival curves of patients in the irAEs-positive group and the irAEs-negative group
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
义维丽, 赵文成, 黄东宁, 等. PD-1单抗治疗非小细胞肺癌相关不良反应及其与疗效的相关性分析[J]. 中国癌症杂志, 2021, 31(3): 203-211. DOI: 10.19401/j.cnki.1007-3639.2021.03.007.
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
中国临床肿瘤学会指南工作委员会组织. 中国临床肿瘤学会(CSCO)免疫检查点抑制剂相关的毒性管理指南-2021[M]. 北京: 人民卫生出版社, 2021: 1-122.
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [1] | 赵倩倩, 张梅, 李艳英, 张艳红, 晏文华, 潘慧, 班博. 生长激素缺乏症的临床特征与重组人生长激素疗效分析:一项十年纵向队列研究[J]. 中国全科医学, 2026, 29(06): 733-740. |
| [2] | 曾明慧, 蒯文涛, 陈林, 韩家鑫, 徐连欣, 葛立颖, 代容容, 宓余强, 徐亮. 2型糖尿病对核苷(酸)类似物治疗慢性乙型肝炎效果的影响研究[J]. 中国全科医学, 2025, 28(35): 4414-4420. |
| [3] | 国家重点研发计划《多类型物理刺激方式对机体功能的影响与机理研究》项目专家组, 中国康复医学会脑功能监测与调控康复专业委员会, 北京神经内科学会脑小血管病专业委员会. 老年失能失智非侵入性神经调控的效应和疗效评价的专家共识[J]. 中国全科医学, 2025, 28(34): 4258-4281. |
| [4] | 杨盈天, 吕乾瑜, 吴茜, 侯信铮, 宋建钧, 叶雪姣, 杨晨艳, 王师菡. 基于"医院-体育馆-社区"康复模式的五体平衡操运动对肥胖相关性高血压的疗效研究:一项随机对照试验[J]. 中国全科医学, 2025, 28(32): 4038-4046. |
| [5] | 智从从, 程一乘, 黄子宸, 王孝龙, 李雪, 郑丽华. 《痔中西医结合诊疗指南(2025版)》临床问题与结局指标的收集与确定[J]. 中国全科医学, 2025, 28(32): 4089-4094. |
| [6] | 郑博月, 付积艺, 吴佳霏, 王珺, 李慧. 卡非佐米治疗多发性骨髓瘤的疗效及安全性研究[J]. 中国全科医学, 2025, 28(30): 3806-3814. |
| [7] | 马文源, 祁烁, 商建伟, 陈晓珩, 李哲, 李会龙, 户蕊, 李璐, 司新颖, 丁治国. 甲状腺功能正常的桥本甲状腺炎证候疗效评价量表的初步研制:基于专家咨询和临床调查[J]. 中国全科医学, 2025, 28(28): 3590-3600. |
| [8] | 肖彩红, 崔瑾, 全菲, 晏明熙, 卢春霞, 陈迎龙. 苗药竹技药灸疗法治疗勃起功能障碍的临床疗效:一项随机对照研究[J]. 中国全科医学, 2025, 28(26): 3300-3306. |
| [9] | 李浩, 李江涛, 刘丹, 王建军. 贝利尤单抗和阿尼鲁单抗及泰它西普治疗系统性红斑狼疮疗效和安全性的网状Meta分析[J]. 中国全科医学, 2025, 28(23): 2924-2933. |
| [10] | 阮万百, 李俊峰, 尹艳梅, 彭磊, 朱克祥. 胰腺癌靶向治疗及免疫治疗的研究新进展[J]. 中国全科医学, 2025, 28(23): 2950-2960. |
| [11] | 马盼盼, 王思静, 游娜, 丁大法, 鲁一兵. Danuglipron与Orforglipron治疗2型糖尿病疗效及安全性的Meta分析[J]. 中国全科医学, 2025, 28(21): 2679-2685. |
| [12] | 刘浏, 徐文航, 吕宾, 范一宏. 维得利珠单抗与乌司奴单抗作为初治生物制剂在中重度活动期克罗恩病患者中的疗效比较研究[J]. 中国全科医学, 2025, 28(08): 948-953. |
| [13] | 宋芬芬, 李胜棉. 基于卡瑞利珠单抗的方案治疗局部晚期及转移性食管癌的真实世界研究[J]. 中国全科医学, 2025, 28(07): 844-852. |
| [14] | 罗思富, 金梦龙, 苏比努尔·居热提, 刘紫阳, 付真彦. 不同民族动脉粥样硬化性心血管疾病患者中等剂量他汀类药物治疗的疗效差异研究[J]. 中国全科医学, 2024, 27(36): 4522-4526. |
| [15] | 檀紫瑞, 申青, 刘俊英, 陈砚凝, 姚继方. 表皮生长因子受体非热点突变型非小细胞肺癌一线应用表皮生长因子受体酪氨酸激酶抑制剂及化疗的疗效对比研究[J]. 中国全科医学, 2024, 27(35): 4426-4434. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||





