中国全科医学 ›› 2025, Vol. 28 ›› Issue (30): 3806-3814.DOI: 10.12114/j.issn.1007-9572.2024.0477
郑博月1, 付积艺1, 吴佳霏1, 王珺2, 李慧1,3,*( )
)
收稿日期:2024-08-29
修回日期:2024-10-18
出版日期:2025-10-20
发布日期:2025-08-18
通讯作者:
李慧
作者贡献:
郑博月、付积艺、吴佳霏、王珺负责数据收集;郑博月、付积艺负责数据分析;郑博月负责论文撰写;付积艺、吴佳霏、王珺负责患者随访;李慧负责研究课题的提出,文章的审查和修订,对文章整体负责。
基金资助:
ZHENG Boyue1, FU Jiyi1, WU Jiafei1, WANG Jun2, LI Hui1,3,*( )
)
Received:2024-08-29
Revised:2024-10-18
Published:2025-10-20
Online:2025-08-18
Contact:
LI Hui
摘要: 背景 卡非佐米自2022年在我国上市以来,已在复发难治性多发性骨髓瘤(RRMM)患者中广泛应用,但目前该药在我国多发性骨髓瘤(MM)患者中的临床有效性及安全性研究不足。 目的 探讨卡非佐米治疗MM的临床疗效与安全性。 方法 纳入2022年3月—2023年9月四川省人民医院接受至少2个疗程卡非佐米治疗的53例MM患者为研究对象,收集患者的基线资料,患者均采用以卡非佐米为基础的方案进行治疗,以研究对象首次使用卡非佐米的时间为随访起点,以死亡、疾病复发或随访结束为随访终点,通过门诊、住院复查或使用电话对患者进行随访,每2个月随访1次,并记录严格意义的完全缓解(sCR)、完全缓解(CR)、非常好的部分缓解(VGPR)、部分缓解(PR)以及发生不良反应患者例数,计算总缓解率(ORR)、≥VGPR率、最佳ORR和最佳≥VGPR率,评估临床疗效;根据患者的治疗线数、髓外病变、Durie-Salmon(DS)分期、国际分期系统(ISS)、肾功能、心血管疾病进行亚组间疗效分析;采用Kaplan-Meier法绘制患者无进展生存期(PFS)和总生存期(OS)生存曲线进行生存分析,生存曲线的比较采用Log-rank检验。 结果 治疗2个疗程后,53例患者中PR为17例(32.1%),VGPR为11例(20.8%),CR为4例(7.5%),sCR为8例(15.1%),ORR为75.5%(40/53)。总体疗效评估显示,最佳ORR为84.9%(45/53),最佳≥VGPR率为71.7%(38/53)。一线用药组、首次复发用药组与三线及以上用药组总体临床疗效比较,差异无统计学意义(P>0.05);三组患者PFS生存曲线比较,差异有统计学意义(P<0.05);三组患者OS生存曲线比较,差异无统计学意义(P>0.05);mSMART标危组与高危组、DS分期Ⅰ~Ⅲ组、ISS分期Ⅰ~Ⅲ组、有髓外病变组与无髓外病变组、有心血管疾病组与无心血管疾病组、肾功能正常组与异常组之间临床疗效、PFS、OS生存曲线比较,差异均无统计学意义(P>0.05)。53例患者中感染患者8例(15.1%),血压升高、心律失常、心衰加重等不良反应患者7例(1.2%),恶心、呕吐等胃肠道不良反应患者3例(5.7%),肝功能损害患者1例(1.9%),肾功能损害患者1例(1.9%)。不良反应发生率为37.7%(20/53)。 结论 以卡非佐米为基础的化疗方案具有较好的临床疗效,且安全性高,可作为多发性骨髓瘤患者治疗的优选方案。
中图分类号:
| 联合用药方案 | 例数(例) |
|---|---|
| 卡非佐米+来那度胺+地塞米松(KRd) | 26 |
| 卡非佐米+泊马度胺+地塞米松(KPd) | 14 |
| 卡非佐米+环磷酰胺+地塞米松(KCd) | 3 |
| 达雷妥尤单抗+卡非佐米+地塞米松(DKd) | 3 |
| 卡非佐米+地塞米松(Kd) | 3 |
| 卡非佐米+地塞米松+顺铂+多柔比星脂质体+依托泊苷+环磷酰胺(Kd-PACE) | 2 |
| 卡非佐米+苯达莫司汀+地塞米松(KBd) | 1 |
| 达雷妥尤单抗+卡非佐米+来那度胺+地塞米松(DKRd) | 1 |
表1 患者联合用药方案
Table 1 Combination regimen for patients
| 联合用药方案 | 例数(例) |
|---|---|
| 卡非佐米+来那度胺+地塞米松(KRd) | 26 |
| 卡非佐米+泊马度胺+地塞米松(KPd) | 14 |
| 卡非佐米+环磷酰胺+地塞米松(KCd) | 3 |
| 达雷妥尤单抗+卡非佐米+地塞米松(DKd) | 3 |
| 卡非佐米+地塞米松(Kd) | 3 |
| 卡非佐米+地塞米松+顺铂+多柔比星脂质体+依托泊苷+环磷酰胺(Kd-PACE) | 2 |
| 卡非佐米+苯达莫司汀+地塞米松(KBd) | 1 |
| 达雷妥尤单抗+卡非佐米+来那度胺+地塞米松(DKRd) | 1 |
| 分组 | 例数 | 性别(男/女) | χ2值 | P值 |
|---|---|---|---|---|
| 治疗线数 | 1.385 | 0.709 | ||
| 一线用药 | 14 | 6/8 | ||
| 首次复发用药 | 20 | 7/13 | ||
| 三线及以上用药 | 14 | 6/8 | ||
| 髓外病变 | 0.050 | 0.823 | ||
| 有 | 17 | 7/10 | ||
| 无 | 36 | 16/20 | ||
| DS分期 | 0.618 | 0.734 | ||
| Ⅰ期 | 11 | 5/6 | ||
| Ⅱ期 | 10 | 5/5 | ||
| Ⅲ期 | 32 | 13/19 | ||
| ISS分期 | 0.686 | 0.101 | ||
| Ⅰ期 | 14 | 10/4 | ||
| Ⅱ期 | 11 | 2/9 | ||
| Ⅲ期 | 28 | 11/17 | ||
| mSMART | 0.325 | 0.561 | ||
| 标危型 | 23 | 11/12 | ||
| 高危型 | 30 | 12/18 | ||
| 肾功能 | 0.091 | 0.763 | ||
| 正常 | 15 | 7/8 | ||
| 异常 | 38 | 16/22 | ||
| 心血管疾病 | 0.484 | 0.487 | ||
| 无 | 35 | 14/21 | ||
| 有 | 18 | 9/9 |
表2 不同治疗线数、髓外病变、分期及基础疾病患者的性别比较
Table 2 Comparison of sex of patients with different treatment lines,extramedullary disease,stage and basic diseases
| 分组 | 例数 | 性别(男/女) | χ2值 | P值 |
|---|---|---|---|---|
| 治疗线数 | 1.385 | 0.709 | ||
| 一线用药 | 14 | 6/8 | ||
| 首次复发用药 | 20 | 7/13 | ||
| 三线及以上用药 | 14 | 6/8 | ||
| 髓外病变 | 0.050 | 0.823 | ||
| 有 | 17 | 7/10 | ||
| 无 | 36 | 16/20 | ||
| DS分期 | 0.618 | 0.734 | ||
| Ⅰ期 | 11 | 5/6 | ||
| Ⅱ期 | 10 | 5/5 | ||
| Ⅲ期 | 32 | 13/19 | ||
| ISS分期 | 0.686 | 0.101 | ||
| Ⅰ期 | 14 | 10/4 | ||
| Ⅱ期 | 11 | 2/9 | ||
| Ⅲ期 | 28 | 11/17 | ||
| mSMART | 0.325 | 0.561 | ||
| 标危型 | 23 | 11/12 | ||
| 高危型 | 30 | 12/18 | ||
| 肾功能 | 0.091 | 0.763 | ||
| 正常 | 15 | 7/8 | ||
| 异常 | 38 | 16/22 | ||
| 心血管疾病 | 0.484 | 0.487 | ||
| 无 | 35 | 14/21 | ||
| 有 | 18 | 9/9 |
| 分组 | 例数 | 年龄(岁) | Z(F)值 | P值 |
|---|---|---|---|---|
| 治疗线数( | 0.37a | 0.105 | ||
| 一线用药 | 14 | 56.9±14.3 | ||
| 首次复发用药 | 20 | 6.8±8.2 | ||
| 三线及以上用药 | 14 | 64.7±9.4 | ||
| 髓外病变[M(P25,P75)] | -1.41 | 0.158 | ||
| 有 | 17 | 66.0(59.0,71.2) | ||
| 无 | 36 | 60.0(55.0,69.0) | ||
| DS分期[M(P25,P75)] | 5.68 | 0.058 | ||
| Ⅰ期 | 11 | 69.0(6.0,76.0) | ||
| Ⅱ期 | 10 | 57.0(56.0,62.0) | ||
| Ⅲ期 | 32 | 65.0(58.0,70.0) | ||
| ISS分期[M(P25,P75)] | 1.21 | 0.545 | ||
| Ⅰ期 | 14 | 67.0(56.0,70.0) | ||
| Ⅱ期 | 11 | 68.0(60.0,75.5) | ||
| Ⅲ期 | 28 | 6.5(57.8,68.2) | ||
| mSMART[M(P25,P75)] | 0.96 | 0.337 | ||
| 标危型 | 23 | 69.0(61.0,71.0) | ||
| 高危型 | 30 | 60.5(57.0,69.8) | ||
| 肾功能[M(P25,P75)] | 0.38 | 0.707 | ||
| 正常 | 38 | 60.0(58.0,69.0) | ||
| 异常 | 15 | 66.0(57.5,70.0) | ||
| 心血管疾病[M(P25,P75)] | 1.61 | 0.108 | ||
| 无 | 35 | 59.5(55.5,67.8) | ||
| 有 | 18 | 66.0(60.0,70.5) | ||
表3 不同治疗线数、髓外病变、分期及基础疾病患者的年龄比较
Table 3 Comparison age of patients with different treatment lines,extramedullary disease,stage and basic diseases
| 分组 | 例数 | 年龄(岁) | Z(F)值 | P值 |
|---|---|---|---|---|
| 治疗线数( | 0.37a | 0.105 | ||
| 一线用药 | 14 | 56.9±14.3 | ||
| 首次复发用药 | 20 | 6.8±8.2 | ||
| 三线及以上用药 | 14 | 64.7±9.4 | ||
| 髓外病变[M(P25,P75)] | -1.41 | 0.158 | ||
| 有 | 17 | 66.0(59.0,71.2) | ||
| 无 | 36 | 60.0(55.0,69.0) | ||
| DS分期[M(P25,P75)] | 5.68 | 0.058 | ||
| Ⅰ期 | 11 | 69.0(6.0,76.0) | ||
| Ⅱ期 | 10 | 57.0(56.0,62.0) | ||
| Ⅲ期 | 32 | 65.0(58.0,70.0) | ||
| ISS分期[M(P25,P75)] | 1.21 | 0.545 | ||
| Ⅰ期 | 14 | 67.0(56.0,70.0) | ||
| Ⅱ期 | 11 | 68.0(60.0,75.5) | ||
| Ⅲ期 | 28 | 6.5(57.8,68.2) | ||
| mSMART[M(P25,P75)] | 0.96 | 0.337 | ||
| 标危型 | 23 | 69.0(61.0,71.0) | ||
| 高危型 | 30 | 60.5(57.0,69.8) | ||
| 肾功能[M(P25,P75)] | 0.38 | 0.707 | ||
| 正常 | 38 | 60.0(58.0,69.0) | ||
| 异常 | 15 | 66.0(57.5,70.0) | ||
| 心血管疾病[M(P25,P75)] | 1.61 | 0.108 | ||
| 无 | 35 | 59.5(55.5,67.8) | ||
| 有 | 18 | 66.0(60.0,70.5) | ||
| 组别 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| 一线用药 | 14 | 5(35.7) | 3(21.4) | 2(14.3) | 2(14.3) | 50.0(7/14) | 85.7(12/14) |
| 首次复发用药 | 20 | 4(20.0) | 5(25.0) | 1(5.0) | 6(30.0) | 60.0(12/20) | 80.0(16/20) |
| 三线及以上用药 | 14 | 4(28.6) | 3(21.4) | 1(7.1) | 0(0.0) | 28.6(4/14) | 57.1(8/14) |
表4 不同治疗线数患者临床疗效
Table 4 Clinical efficacy of different treatment lines
| 组别 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| 一线用药 | 14 | 5(35.7) | 3(21.4) | 2(14.3) | 2(14.3) | 50.0(7/14) | 85.7(12/14) |
| 首次复发用药 | 20 | 4(20.0) | 5(25.0) | 1(5.0) | 6(30.0) | 60.0(12/20) | 80.0(16/20) |
| 三线及以上用药 | 14 | 4(28.6) | 3(21.4) | 1(7.1) | 0(0.0) | 28.6(4/14) | 57.1(8/14) |
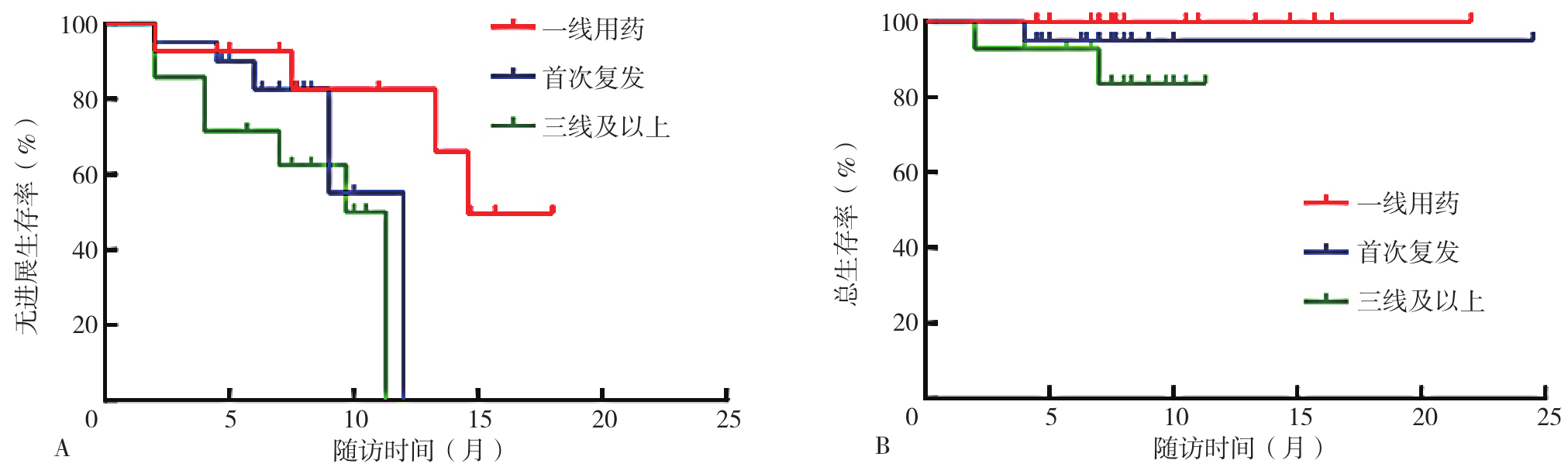
图1 一线用药、首次复发用药、三线及以上用药患者生存曲线注:A为一线用药、首次复发用药、三线及以上用药患者PFS生存曲线,B为一线用药、首次复发用药、三线及以上用药患者OS生存曲线。
Figure 1 Survival curve of patients with first-line treatment,first-time relapse treatment and third-line and above drugs
| 组别 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| 无髓外病变 | 36 | 12(0.3) | 9(25.0) | 1(0.8) | 5(1.9) | 41.7(15/36) | 75.0(27/36) |
| 有髓外病变 | 17 | 5(29.4) | 2(11.8) | 3(17.6) | 3(17.6) | 47.1(8/17) | 76.4(13/17) |
表5 有髓外病变与无髓外病变患者临床疗效
Table 5 Clinical efficacy of patients with and without extramedullary disease
| 组别 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| 无髓外病变 | 36 | 12(0.3) | 9(25.0) | 1(0.8) | 5(1.9) | 41.7(15/36) | 75.0(27/36) |
| 有髓外病变 | 17 | 5(29.4) | 2(11.8) | 3(17.6) | 3(17.6) | 47.1(8/17) | 76.4(13/17) |
| mSMART分期 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| 标危型 | 12 | 0 | 3(25.0) | 1(8.3) | 6(50.0) | 83.3(10/12) | 83.3(10/12) |
| 高危型 | 41 | 17(41.5) | 8(19.5) | 3(7.3) | 2(4.9) | 31.7(13/41) | 73.2(30/41) |
表6 mSMART高危型与标危型患者临床疗效
Table 6 Clinical efficacy of high risk and standard riskof mSMART types
| mSMART分期 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| 标危型 | 12 | 0 | 3(25.0) | 1(8.3) | 6(50.0) | 83.3(10/12) | 83.3(10/12) |
| 高危型 | 41 | 17(41.5) | 8(19.5) | 3(7.3) | 2(4.9) | 31.7(13/41) | 73.2(30/41) |
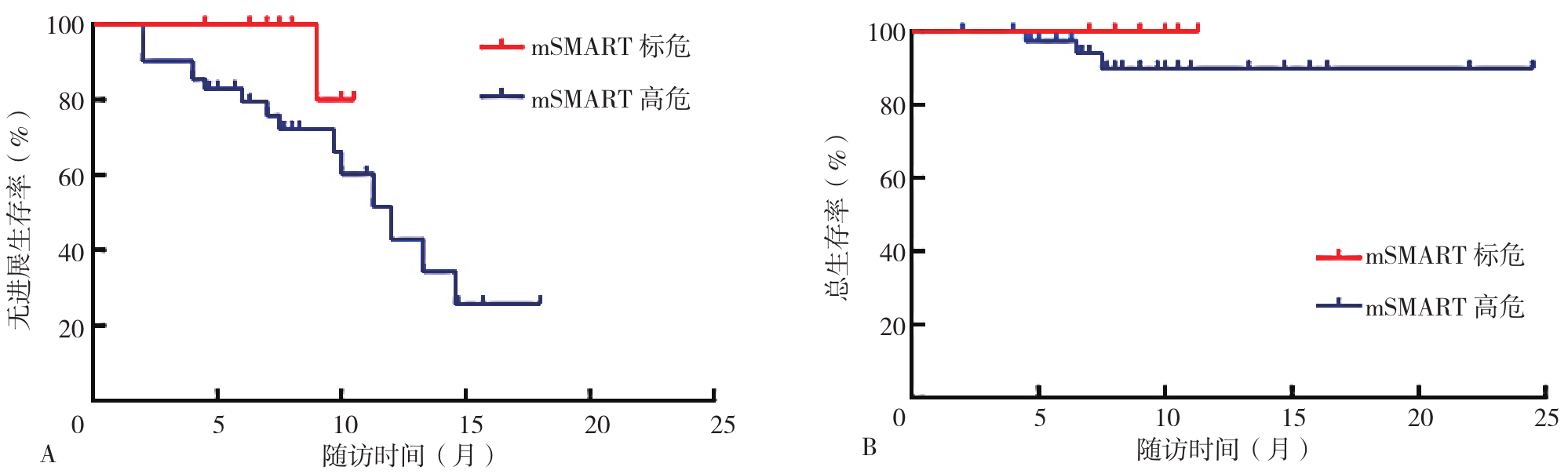
图3 mSMART高危型与mAMART标危型患者生存曲线注:mSMART=梅奥多发性骨髓瘤3.0危险分层系统,A为mSMART高危型与标危型患者PFS生存曲线,B为mSMART高危型与标危型患者OS生存曲线。
Figure 3 Survival curves for patients with high risk and standard risk of mSMART types
| 组别 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| Ⅰ期 | 11 | 3(27.3) | 2(18.2) | 1(9.1) | 3(27.3) | 54.5(6/11) | 81.8(9/11) |
| Ⅱ期 | 10 | 2(20.0) | 4(40.0) | 1(10.0) | 0 | 50.0(5/10) | 70.0(7/10) |
| Ⅲ期 | 32 | 12(37.5) | 5(15.6) | 2(6.3) | 5(15.6) | 37.5(12/32) | 75.0(24/32) |
表7 DS Ⅰ~Ⅲ期患者临床疗效
Table 7 Clinical efficacy of patients with DS stage Ⅰ-Ⅲ
| 组别 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| Ⅰ期 | 11 | 3(27.3) | 2(18.2) | 1(9.1) | 3(27.3) | 54.5(6/11) | 81.8(9/11) |
| Ⅱ期 | 10 | 2(20.0) | 4(40.0) | 1(10.0) | 0 | 50.0(5/10) | 70.0(7/10) |
| Ⅲ期 | 32 | 12(37.5) | 5(15.6) | 2(6.3) | 5(15.6) | 37.5(12/32) | 75.0(24/32) |
| 组别 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| Ⅰ期 | 14 | 4(28.6) | 1(7.1) | 1(7.1) | 3(21.4) | 35.7(5/14) | 64.2(9/14) |
| Ⅱ期 | 11 | 5(45.5) | 2(18.2) | 0 | 3(27.3) | 45.5(5/11) | 90.9(10/11) |
| Ⅲ期 | 28 | 8(28.6) | 8(28.6) | 3(10.7) | 2(7.1) | 46.4(13/28) | 75.0(21/28) |
表8 ISS Ⅰ~Ⅲ期患者临床疗效
Table 8 Clinical efficacy among patients with different ISS stage Ⅰ-Ⅲ
| 组别 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| Ⅰ期 | 14 | 4(28.6) | 1(7.1) | 1(7.1) | 3(21.4) | 35.7(5/14) | 64.2(9/14) |
| Ⅱ期 | 11 | 5(45.5) | 2(18.2) | 0 | 3(27.3) | 45.5(5/11) | 90.9(10/11) |
| Ⅲ期 | 28 | 8(28.6) | 8(28.6) | 3(10.7) | 2(7.1) | 46.4(13/28) | 75.0(21/28) |
| 组别 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| 无心血管疾病 | 35 | 10(28.6) | 10(28.6) | 2(5.7) | 5(14.3) | 48.6(17/35) | 77.1(27/35) |
| 有心血管疾病 | 18 | 7(38.9) | 1(5.6) | 2(11.1) | 3(16.7) | 27.8(5/18) | 66.6(12/18) |
表9 有心血管疾病组与无心血管疾病组临床疗效
Table 9 Clinical efficacy analysis between patients with and without cardiovascular disease
| 组别 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| 无心血管疾病 | 35 | 10(28.6) | 10(28.6) | 2(5.7) | 5(14.3) | 48.6(17/35) | 77.1(27/35) |
| 有心血管疾病 | 18 | 7(38.9) | 1(5.6) | 2(11.1) | 3(16.7) | 27.8(5/18) | 66.6(12/18) |
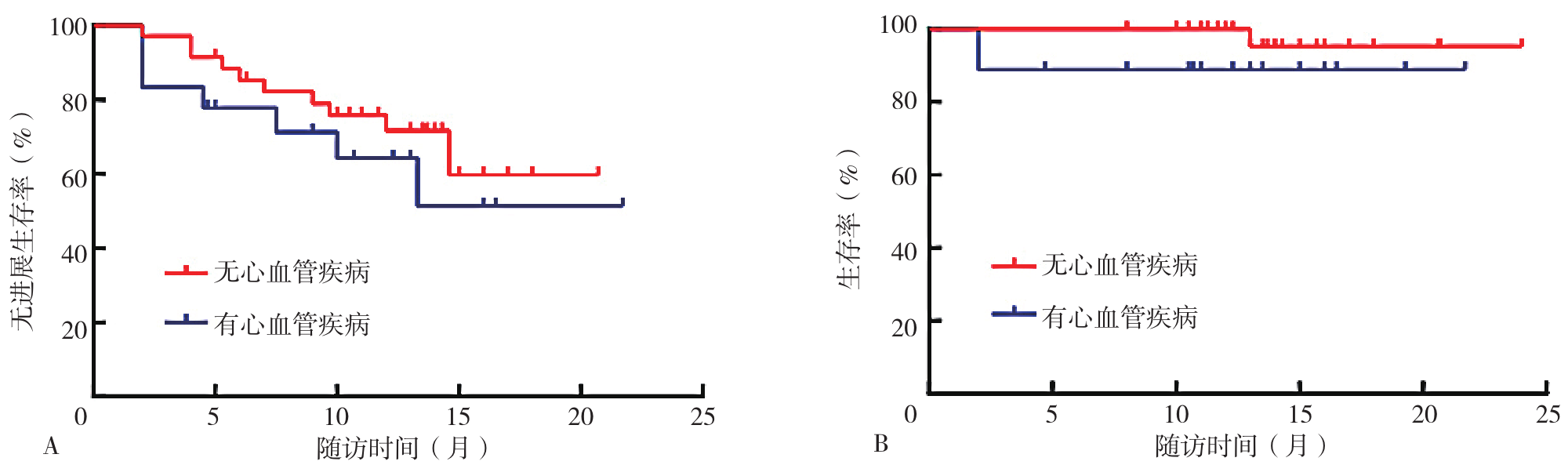
图6 有心血管疾病患者与无心血管疾病患者生存曲线注:A为有心血管疾病患者与无心血管疾病患者PFS生存曲线,B为有心血管疾病患者与无心血管疾病患者OS生存曲线。
Figure 6 Survival curves for patients with and without cardiovascular disease
| 组别 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| 肾功能正常组 | 38 | 14(35.8) | 7(18.4) | 3(7.9) | 7(18.4) | 44.7(17/38) | 81.6(31/38) |
| 肾功能异常组 | 15 | 3(20.0) | 4(26.7) | 1(6.7) | 1(66.7) | 40.0(6/15) | 60%(9/15) |
表10 肾功能正常组与肾功能异常组临床疗效
Table 10 Clinical efficacy analysis between the normal renal function group and the abnormal renal function group
| 组别 | 例数 | PR[例(%)] | VGPR[例(%)] | CR[例(%)] | sCR[例(%)] | ≥VGPR率[%(n/n)] | ORR[%(n/n)] |
|---|---|---|---|---|---|---|---|
| 肾功能正常组 | 38 | 14(35.8) | 7(18.4) | 3(7.9) | 7(18.4) | 44.7(17/38) | 81.6(31/38) |
| 肾功能异常组 | 15 | 3(20.0) | 4(26.7) | 1(6.7) | 1(66.7) | 40.0(6/15) | 60%(9/15) |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
中国医师协会血液科医师分会,中华医学会血液学分会,黄晓军. 中国多发性骨髓瘤诊治指南(2022年修订)[J]. 中华内科杂志,2022,61(5):480-487. DOI:10.3760/cma.j.cn112138-20220309-00165.
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [1] | 武向鹏, 李恩君, 李雄伟, 王海红, 崔薇, 武向丽, 祁卫华, 侯森林. 非甾体抗炎药在内镜逆行胰胆管造影术后胰腺炎预防中的给药时机和影响因素研究[J]. 中国全科医学, 2025, 28(30): 3823-3830. |
| [2] | 张天宇, 于海搏, 陈飞, 李新, 张佳佳, 詹晓凯, 申曼, 汤然, 范斯斌, 赵凤仪, 黄仲夏. POEMS综合征全身系统性治疗疗效和安全性的Meta分析[J]. 中国全科医学, 2025, 28(27): 3447-3455. |
| [3] | 陈飞, 王金英, 于海搏, 李新, 张佳佳, 申曼, 詹晓凯, 汤然, 范斯斌, 赵凤仪, 张天宇, 黄仲夏. 中性粒细胞明胶酶相关运载蛋白、T细胞免疫球蛋白粘蛋白受体1、血管细胞黏附分子-1和激活素A升高在新诊断多发性骨髓瘤患者中的意义研究[J]. 中国全科医学, 2025, 28(22): 2740-2749. |
| [4] | 马湖萍, 任蓉, 侯梅, 苑爱云. 新型抗癫痫发作药物吡仑帕奈添加治疗0~18岁儿童难治性癫痫的临床观察研究[J]. 中国全科医学, 2025, 28(02): 250-256. |
| [5] | 吴凯瑞, 叶宇, 李娇月, 裴蓓, 李学军, 程红亮. 脾胃培源方加减联合针刺治疗慢性萎缩性胃炎伴肠化生效果的多中心临床随机对照试验[J]. 中国全科医学, 2024, 27(20): 2466-2475. |
| [6] | 谭书法, 张磊昌, 高强强, 欧艳, 黄水兰. 生物制剂和小分子药物治疗溃疡性结肠炎有效性与安全性的网状Meta分析[J]. 中国全科医学, 2024, 27(17): 2155-2166. |
| [7] | 赵慧, 李文豪, 程功, 陈亮, 梁宸源, 王依阳, 蒋红英, 姜瑞嘉. 腺苷负荷与ATP负荷评估冠状动脉微血管疾病中的不良反应分析[J]. 中国全科医学, 2024, 27(17): 2109-2112. |
| [8] | 白鑫, 武新宇, 赵尊, 柳舒心, 刘斯淼, 薛宇航, 徐俊玲, 高永举. 131I治疗血清甲状腺球蛋白抗体阳性分化型甲状腺癌远处转移的疗效研究[J]. 中国全科医学, 2024, 27(15): 1833-1837. |
| [9] | 赵凤仪, 李新, 詹晓凯, 张佳佳, 申曼, 汤然, 范斯斌, 黄仲夏. 初诊超高龄多发性骨髓瘤患者硼替佐米基础方案治疗的生存预后分析[J]. 中国全科医学, 2024, 27(08): 971-977. |
| [10] | 于海搏, 张天宇, 李新, 张佳佳, 申曼, 詹晓凯, 汤然, 范斯斌, 赵凤仪, 黄仲夏. 双靶点嵌合抗原受体-T细胞治疗复发难治多发性骨髓瘤患者疗效和安全性的Meta分析[J]. 中国全科医学, 2024, 27(08): 985-994. |
| [11] | 彭逸伦, 李杨, 王晓桃. 多发性骨髓瘤细胞通过PI3K/AKT信号通路促进M2巨噬细胞极化的机制研究[J]. 中国全科医学, 2024, 27(08): 978-984. |
| [12] | 张翼升, 唐福波, 孙亚如, 钟远鸣, 李智斐. 经皮内镜后路经椎间孔腰椎椎间融合术联合高度可调钛质融合器治疗腰椎滑脱合并腰椎管狭窄症的临床疗效分析[J]. 中国全科医学, 2023, 26(35): 4464-4471. |
| [13] | 李茜, 张云淑, 严保平, 王健, 马燕娟, 王媛, 秦英杰, 那龙, 任智勇, 孙俊伟, 邓怀丽, 马宏筠, 曲雪慧, 周楠, 司天梅. 注射用利培酮微球(Ⅱ)治疗急性期精神分裂症患者的疗效及安全性研究[J]. 中国全科医学, 2023, 26(32): 4007-4012. |
| [14] | 王珺, 吴佳霏, 王依景, 郑博月, 王宇, 江川艳, 李慧. 以达雷妥尤单抗为基础的化疗方案对多发性骨髓瘤疗效和预后影响的真实世界研究[J]. 中国全科医学, 2023, 26(18): 2256-2262. |
| [15] | 陈璐璐, 张利苹, 李静文, 董文杰, 吴欣爱. 程序性死亡受体1抑制剂联合呋喹替尼后线治疗转移性结直肠癌的临床疗效和安全性研究[J]. 中国全科医学, 2023, 26(18): 2262-2267. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||





