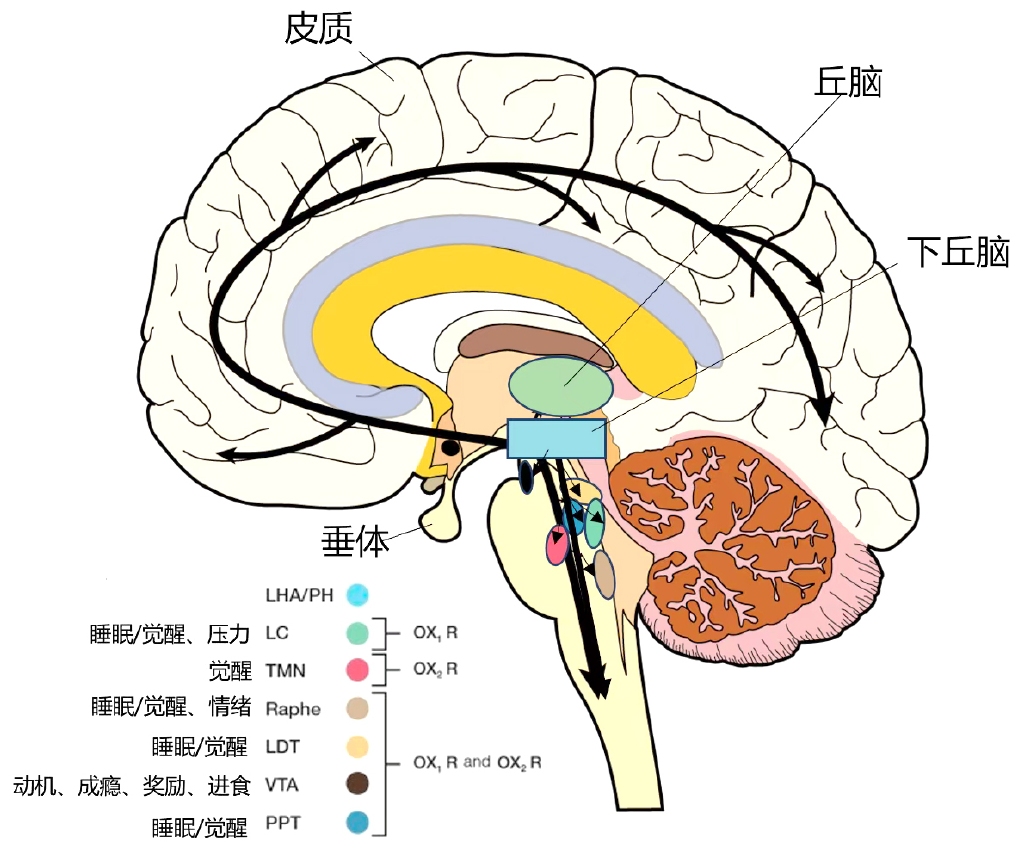
| [1] |
张斌. 中国失眠障碍诊断和治疗指南[M]. 2版. 北京: 人民卫生出版社, 2025: 147.
|
| [2] |
HOC T V, LEE H C. Clinical and polysomnographic characteristics of Asian patients with comorbid insomnia and obstructive sleep apnea[J]. Sci Rep, 2025, 15(1): 11529. DOI: 10.1038/s41598-025-96825-7.
|
| [3] |
HERTENSTEIN E, BENZ F, SCHNEIDER C L, et al. Insomnia-a risk factor for mental disorders[J]. J Sleep Res, 2023, 32(6): e13930. DOI: 10.1111/jsr.13930.
|
| [4] |
KRYSTAL A D. Insomnia medications: history, characteristics, and guidelines for optimal use in clinical practice[J]. J Sleep Res, 2023, 32(6): e14084. DOI: 10.1111/jsr.14084.
|
| [5] |
MOLINE M, ZAMMIT G, YARDLEY J, et al. Lack of residual morning effects of lemborexant treatment for insomnia: summary of findings across 9 clinical trials[J]. Postgrad Med, 2021, 133(1): 71-81. DOI: 10.1080/00325481.2020.1823724.
|
| [6] |
LI X, ZHENG S, JIAN W, et al. Reported OUTCOMES for LEMborexant treatment in Chinese patients with insomnia(PROEM): a real-world study[C]//SLEEP 2025, the 39th Annual Meeting of the Associated Professional Sleep Societies, LLC(APSS). Seattle, Washington, USA, 2025.
|
| [7] |
|
| [8] |
CHIEFFI S, CAROTENUTO M, MONDA V, et al. Orexin system: the key for a healthy life[J]. Front Physiol, 2017, 8: 357. DOI: 10.3389/fphys.2017.00357.
|
| [9] |
NEVÁREZ N, DE LECEA L. Recent advances in understanding the roles of hypocretin/orexin in arousal, affect, and motivation[J]. F1000Res, 2018, 7: F1000FacultyRev-F1000Faculty1421. DOI: 10.12688/f1000research.15097.1.
|
| [10] |
ARDELJAN A, HUREZEANU R. Lemborexant[J]. Am J Health Syst Pharm, 2020, 77(17): 1358-1361.
|
| [11] |
HOYER D, ALLEN A, JACOBSON L H. Hypnotics with novel modes of action[J]. Br J Clin Pharmacol, 2020, 86(2): 244-249. DOI: 10.1111/bcp.14180.
|
| [12] |
FRONCZEK R, LAMMERS G J, BALESAR R, et al. The number of hypothalamic hypocretin (orexin) neurons is not affected in Prader-Willi syndrome[J]. J Clin Endocrinol Metab, 2005, 90(9): 5466-5470. DOI: 10.1210/jc.2005-0296.
|
| [13] |
THANNICKAL T C, MOORE R Y, NIENHUIS R, et al. Reduced number of hypocretin neurons in human narcolepsy[J]. Neuron, 2000, 27(3): 469-474. DOI: 10.1016/s0896-6273(00)00058-1.
|
| [14] |
MIEDA M, HASEGAWA E, KISANUKI Y Y, et al. Differential roles of orexin receptor-1 and-2 in the regulation of non-REM and REM sleep[J]. J Neurosci, 2011, 31(17): 6518-6526. DOI: 10.1523/JNEUROSCI.6506-10.2011.
|
| [15] |
PEYRON C, FARACO J, ROGERS W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains[J]. Nat Med, 2000, 6(9): 991-997. DOI: 10.1038/79690.
|
| [16] |
FULLER M C, CARLSON S F, GRANT C, et al. A comprehensive review of lemborexant to treat insomnia[J]. Psychopharmacol Bull, 2024, 54(1): 43-64.
|
| [17] |
COLEMAN P J, GOTTER A L, JOSEPH HERRING W, et al. The discovery of suvorexant, the first orexin receptor drug for insomnia[J]. Annu Rev Pharmacol Toxicol, 2017, 57: 509-533. DOI: 10.1146/annurev-pharmtox-010716-104837.
|
| [18] |
BEUCKMANN C T, SUZUKI M, UENO T, et al. In vitro and in silico characterization of lemborexant (E2006), a novel dual orexin receptor antagonist[J]. J Pharmacol Exp Ther, 2017, 362(2): 287-295. DOI: 10.1124/jpet.117.241422.
|
| [19] |
SAKURAI T, AMEMIYA A, ISHII M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior[J]. Cell, 1998, 92(4): 573-585. DOI: 10.1016/s0092-8674(00)80949-6.
|
| [20] |
KISHI T, NISHIDA M, KOEBIS M, et al. Evidence-based insomnia treatment strategy using novel orexin antagonists: a review[J]. Neuropsychopharmacol Rep, 2021, 41(4): 450-458. DOI: 10.1002/npr2.12205.
|
| [21] |
KISHI T, NOMURA I, MATSUDA Y, et al. Lemborexant vs suvorexant for insomnia: a systematic review and network meta-analysis[J]. J Psychiatr Res, 2020, 128: 68-74. DOI: 10.1016/j.jpsychires.2020.05.025.
|
| [22] |
FDA. DAYVIGO (Lemborexant): highlights of prescribing information[EB/OL]. [2025-05-08].
|
| [23] |
LANDRY I, HALL N, ALURI J, et al. Effect of alcohol coadministration on the pharmacodynamics, pharmacokinetics, and safety of lemborexant: a randomized, placebo-controlled crossover study[J]. J Psychopharmacol, 2022, 36(6): 745-755. DOI: 10.1177/02698811221080459.
|
| [24] |
LALOVIC B, MAJID O, ALURI J, et al. Population pharmacokinetics and exposure-response analyses for the most frequent adverse events following treatment with lemborexant, an orexin receptor antagonist, in subjects with insomnia disorder[J]. J Clin Pharmacol, 2020, 60(12): 1642-1654. DOI: 10.1002/jcph.1683.
|
| [25] |
LANDRY I, NAKAI K Y, FERRY J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults[J]. Clin Pharmacol Drug Dev, 2021, 10(2): 153-165. DOI: 10.1002/cpdd.817.
|
| [26] |
MANO Y, UENO T, HOTTA K. Establishment of a simultaneous assay for lemborexant, a novel dual orexin receptor antagonist, and its three metabolites, and its application to a clinical protein binding study[J]. J Pharm Biomed Anal, 2020, 187: 113359. DOI: 10.1016/j.jpba.2020.113359.
|
| [27] |
UENO T, ISHIDA T, ALURI J, et al. Disposition and metabolism of [14C] lemborexant in healthy human subjects and characterization of its circulating metabolites[J]. Drug Metab Dispos, 2021, 49(1): 31-38. DOI: 10.1124/dmd.120.000229.
|
| [28] |
ROSENBERG R, MURPHY P, ZAMMIT G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial[J]. JAMA Netw Open, 2019, 2(12): e1918254. DOI: 10.1001/jamanetworkopen.2019.18254.
|
| [29] |
MOLINE M, ZAMMIT G, CHENG J Y, et al. Comparison of the effect of lemborexant with placebo and zolpidem tartrate extended release on sleep architecture in older adults with insomnia disorder[J]. J Clin Sleep Med, 2021, 17(6): 1167-1174. DOI: 10.5664/jcsm.9150.
|
| [30] |
MCELROY H, O'LEARY B, ADENA M, et al. Comparative efficacy of lemborexant and other insomnia treatments: a network meta-analysis[J]. J Manag Care Spec Pharm, 2021, 27(9): 1296-1308. DOI: 10.18553/jmcp.2021.21011.
|
| [31] |
YUE J L, CHANG X W, ZHENG J W, et al. Efficacy and tolerability of pharmacological treatments for insomnia in adults: a systematic review and network meta-analysis[J]. Sleep Med Rev, 2023, 68: 101746. DOI: 10.1016/j.smrv.2023.101746.
|
| [32] |
MIYATA S, IWAMOTO K, OKADA I, et al. Assessing the real-world, long-term impact of lemborexant on sleep quality in a home-based clinical study[J]. Nat Sci Sleep, 2024, 16: 291-303. DOI: 10.2147/NSS.S448871.
|
| [33] |
TERAUCHI M, CHENG J Y, YARDLEY J, et al. Efficacy and safety of lemborexant in midlife women with insomnia disorder[J]. Menopause, 2023, 30(8): 839-848. DOI: 10.1097/GME.0000000000002209.
|
| [34] |
INOUE Y, WATANABE T, TAKASHIMA S, et al. Efficacy and safety of lemborexant in adults with insomnia: comparing Japanese and non-Japanese subgroups from the global, phase 3, randomized, double-blind, placebo-controlled SUNRISE 2 study[J]. J Clin Sleep Med, 2021, 17(5): 1067-1074. DOI: 10.5664/jcsm.9148.
|
| [35] |
ARNOLD V, ANCOLI-ISRAEL S, DANG-VU T T, et al. Efficacy of lemborexant in adults ≥ 65 years of age with insomnia disorder[J]. Neurol Ther, 2024, 13(4): 1081-1098. DOI: 10.1007/s40120-024-00622-9.
|
| [36] |
KÄRPPÄ M, YARDLEY J, PINNER K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2[J]. Sleep, 2020, 43(9): zsaa123. DOI: 10.1093/sleep/zsaa123.
|
| [37] |
KRYSTAL A, BLIER P, CULPEPPER L, et al. Efficacy and safety of lemborexant in subjects with insomnia disorder receiving medications for depression or anxiety symptoms[J]. Neuropsychopharmacol Rep, 2025, 45(1): e12509. DOI: 10.1002/npr2.12509.
|
| [38] |
MURAYAMA T, ITO Y, NARITA K, et al. The effect of lemborexant on insomnia in patients with psychiatric disorders: detailed evaluation using the Athens Insomnia Scale[J]. PCN Rep, 2024, 3(1): e165. DOI: 10.1002/pcn5.165.
|
| [39] |
KATSUTA N, TAKAHASHI K, KUROSAWA Y, et al. Safety and real-world efficacy of lemborexant in the treatment of comorbid insomnia[J]. Sleep Med X, 2023, 5: 100070. DOI: 10.1016/j.sleepx.2023.100070.
|
| [40] |
HORIKOSHI S, MIURA I, SUZUKI Y, et al. Switching to lemborexant for the management of insomnia in mental disorders: the SLIM study[J]. J Clin Sleep Med, 2023, 19(10): 1753-1758. DOI: 10.5664/jcsm.10668.
|
| [41] |
JIAN W Y, FENG M Y, ZHAO Y F, et al. Efficacy and safety of Lemborexant in treating adult patients with insomnia in China: a single-center, retrospective observational study[J]. Front Neurol, 2025, 16: 1495965. DOI: 10.3389/fneur.2025.1495965.
|
| [42] |
KISHI T, KOEBIS M, SUGAWARA M, et al. Orexin receptor antagonists in the treatment of insomnia associated with psychiatric disorders: a systematic review[J]. Transl Psychiatry, 2024, 14(1): 374. DOI: 10.1038/s41398-024-03087-4.
|
| [43] |
UEMATSU T, TOMITA T, OBARA R, et al. Reducing the use of psychotropics in a convalescent rehabilitation ward[J]. Neuropsychopharmacol Rep, 2024, 44(1): 227-233. DOI: 10.1002/npr2.12388.
|
| [44] |
LIGUORI C, NUCCETELLI M, IZZI F, et al. Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-a cerebrospinal-fluid levels in mild cognitive impairment due to Alzheimer's disease[J]. Neurobiol Aging, 2016, 40: 120-126. DOI: 10.1016/j.neurobiolaging.2016.01.007.
|
| [45] |
LIGUORI C, ROMIGI A, NUCCETELLI M, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease[J]. JAMA Neurol, 2014, 71(12): 1498-1505. DOI: 10.1001/jamaneurol.2014.2510.
|
| [46] |
MUSIEK E S, XIONG D D, HOLTZMAN D M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease[J]. Exp Mol Med, 2015, 47(3): e148. DOI: 10.1038/emm.2014.121.
|
| [47] |
DUNCAN M J, FARLOW H, TIRUMALARAJU C, et al. Effects of the dual orexin receptor antagonist DORA-22 on sleep in 5XFAD mice[J]. Alzheimers Dement, 2019, 5: 70-80. DOI: 10.1016/j.trci.2019.01.003.
|
| [48] |
MOLINE M, THEIN S, BSHARAT M, et al. Safety and efficacy of lemborexant in patients with irregular sleep-wake rhythm disorder and Alzheimer's disease dementia: results from a phase 2 randomized clinical trial[J]. J Prev Alzheimers Dis, 2021, 8(1): 7-18. DOI: 10.14283/jpad.2020.69.
|
| [49] |
BAYLAN S, GRIFFITHS S, GRANT N, et al. Incidence and prevalence of post-stroke insomnia: a systematic review and meta-analysis[J]. Sleep Med Rev, 2020, 49: 101222. DOI: 10.1016/j.smrv.2019.101222.
|
| [50] |
WINROW C J, RENGER J J. Discovery and development of orexin receptor antagonists as therapeutics for insomnia[J]. Br J Pharmacol, 2014, 171(2): 283-293. DOI: 10.1111/bph.12261.
|
| [51] |
WINROW C J, GOTTER A L, COX C D, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist[J]. J Neurogenet, 2011, 25(1/2): 52-61. DOI: 10.3109/01677063.2011.566953.
|
| [52] |
KAWADA K, OHTA T, TANAKA K, et al. Addition of suvorexant to ramelteon therapy for improved sleep quality with reduced delirium risk in acute stroke patients[J]. J Stroke Cerebrovasc Dis, 2019, 28(1): 142-148. DOI: 10.1016/j.jstrokecerebrovasdis.2018.09.024.
|
| [53] |
OZONE M, HIROTA S, ARIYOSHI Y, et al. Efficacy and safety of transitioning to lemborexant from Z-drug, suvorexant, and ramelteon in Japanese insomnia patients: an open-label, multicenter study[J]. Adv Ther, 2024, 41(4): 1728-1745. DOI: 10.1007/s12325-024-02811-2.
|
| [54] |
DE CRESCENZO F, D'ALÒ G L, OSTINELLI E G, et al. Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis[J]. Lancet, 2022, 400(10347): 170-184. DOI: 10.1016/S0140-6736(22)00878-9.
|
| [55] |
TERADA T, HIRAYAMA T, SADAHIRO R, et al. Pilot study of lemborexant for insomnia in cancer patients with delirium[J]. J Palliat Med, 2022, 25(5): 797-801. DOI: 10.1089/jpm.2021.0509.
|
| [56] |
OGURA T, UENO S, OKUDA A, et al. Can lemborexant for insomnia prevent delirium in high-risk patients with pancreato-biliary disease after endoscopic procedures under deep sedation?[J]. J Clin Med, 2022, 12(1): 297. DOI: 10.3390/jcm12010297.
|
| [57] |
CHENG J Y, FILIPPOV G, MOLINE M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study[J]. J Sleep Res, 2020, 29(4): e13021. DOI: 10.1111/jsr.13021.
|
| [58] |
CHENG J Y, LORCH D, LOWE A D, et al. A randomized, double-blind, placebo-controlled, crossover study of respiratory safety of lemborexant in moderate to severe obstructive sleep apnea[J]. J Clin Sleep Med, 2024, 20(1): 57-65. DOI: 10.5664/jcsm.10788.
|
| [59] |
CHENG J Y, MOLINE M, ZAMMIT G K, et al. Respiratory safety of lemborexant in healthy subjects: a single-dose, randomized, double-blind, placebo-controlled, crossover study[J]. Clin Drug Investig, 2021, 41(5): 449-457. DOI: 10.1007/s40261-021-01018-5.
|
| [60] |
KUSHIDA C A, ZAMMIT G K, CHENG J Y, et al. Effect of lemborexant on sleep architecture in participants with insomnia disorder and mild obstructive sleep apnea[J]. Sleep Med, 2025, 127: 170-177. DOI: 10.1016/j.sleep.2024.12.023.
|
| [61] |
CHENG J Y, LORCH D, HALL N, et al. Respiratory safety of lemborexant in adult and elderly subjects with moderate-to-severe chronic obstructive pulmonary disease[J]. J Sleep Res, 2025, 34(2): e14334. DOI: 10.1111/jsr.14334.
|
| [62] |
TOGO Y, KAIZUKA Y, NAGASAWA S, et al. Efficacy and safety of lemborexant for insomnia patients with nocturia-a prospective study[J]. Low Urin Tract Symptoms, 2024, 16(6): e12534. DOI: 10.1111/luts.12534.
|
| [63] |
PRESKORN S H. Comparative pharmacology of the 3 marketed dual orexin antagonists-daridorexant, lemborexant, and suvorexant: part 1: pharmacokinetic profiles[J]. J Psychiatr Pract, 2022, 28(6): 478-480. DOI: 10.1097/PRA.0000000000000672.
|
| [64] |
MURPHY P, MOLINE M, MAYLEBEN D, et al. Lemborexant, A dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study[J]. J Clin Sleep Med, 2017, 13(11): 1289-1299. DOI: 10.5664/jcsm.6800.
|
| [65] |
HABIBA U, WASEEM R, SHAIKH T G, et al. Comparative efficacy and safety of lemborexant 5 mg versus 10 mg for the treatment of insomnia: a systematic review[J]. Neurol Sci, 2023, 44(5): 1533-1541. DOI: 10.1007/s10072-023-06601-6.
|
| [66] |
LANDRY I, ALURI J, NAKAI K Y, et al. Evaluation of the CYP3A and CYP2B6 drug-drug interaction potential of lemborexant[J]. Clin Pharmacol Drug Dev, 2021, 10(6): 681-690. DOI: 10.1002/cpdd.915.
|
| [67] |
UENO T, MIYAJIMA Y, LANDRY I, et al. Physiologically-based pharmacokinetic modeling to predict drug interactions of lemborexant with CYP3A inhibitors[J]. CPT Pharmacometrics Syst Pharmacol, 2021, 10(5): 455-466. DOI: 10.1002/psp4.12606.
|
| [68] |
WATANABE K, MISAKA S, KANNO-NOZAKI K, et al. Effect of lemborexant on pharmacokinetics of clozapine: a potential drug-drug interaction mediated by time-dependent inhibition of CYP3A4[J]. Br J Clin Pharmacol, 2024, 90(1): 354-359. DOI: 10.1111/bcp.15889.
|
| [69] |
LANDRY I, ALURI J, HALL N, et al. Effect of gastric acid-reducing agents on the pharmacokinetics and efficacy of lemborexant[J]. Pharmacol Res Perspect, 2020, 8(6): e00678. DOI: 10.1002/prp2.678.
|
| [70] |
LANDRY I, ALURI J, HALL N, et al. Effects of lemborexant on the pharmacokinetics of oral contraceptives: results from a phase 1 drug-drug interaction study in healthy females[J]. Clin Pharmacol Drug Dev, 2021, 10(9): 1089-1098. DOI: 10.1002/cpdd.953.
|
| [71] |
OKINO K, SUZUKI H, TOMIOKA H, et al. Efficacy and safety of lemborexant as an alternative drug for patients with insomnia taking gamma-aminobutyric acid-benzodiazepine receptor agonists or suvorexant[J]. Hum Psychopharmacol, 2023, 38(3): e2868. DOI: 10.1002/hup.2868.
|
| [72] |
TAKAESU Y, SAKURAI H, AOKI Y, et al. Treatment strategy for insomnia disorder: Japanese expert consensus[J]. Front Psychiatry, 2023, 14: 1168100. DOI: 10.3389/fpsyt.2023.1168100.
|
| [73] |
TACHIBANA M, KANAHARA N, ODA Y, et al. A retrospective clinical practice study comparing the usefulness of dual-orexin receptor antagonists and a melatonin receptor agonist in patients switching from long-term benzodiazepine receptor agonists[J]. J Clin Sleep Med, 2024, 20(4): 603-613. DOI: 10.5664/jcsm.10946.
|
| [74] |
PALAGINI L, BRUGNOLI R, DELL' OSSO B M, et al. Clinical practice guidelines for switching or deprescribing hypnotic medications for chronic insomnia: results of European Neuropsychopharmacology and Sleep Expert's Consensus Group[J]. Sleep Med, 2025, 128: 117-126. DOI: 10.1016/j.sleep.2025.01.033.
|
| [75] |
HINTZE J P, EDINGER J D. Hypnotic discontinuation in chronic insomnia[J]. Sleep Med Clin, 2020, 15(2): 147-154. DOI: 10.1016/j.jsmc.2020.02.003.
|
| [76] |
AHMAD M, KELLY J, MONTANO C B, et al. Transitioning insomnia patients from zolpidem to lemborexant: a multicenter, open-label study evaluating a next-dose transition approach to insomnia pharmacotherapy[J]. Sleep Med X, 2024, 7: 100098. DOI: 10.1016/j.sleepx.2023.100098.
|
| [77] |
TANAKA-MIZUNO S, FUJIMOTO K, MISHIMA K, et al. Evaluation of prescribing patterns of switching to and add-on lemborexant in patients treated with hypnotic medication: a nationwide claims database study in Japan[J]. Expert Opin Pharmacother, 2024, 25(12): 1707-1716. DOI: 10.1080/14656566.2024.2392018.
|
| [78] |
SUZUKI H, HIBINO H. Characteristics of patients who were able to switch from benzodiazepine hypnotics to lemborexant[J]. SAGE Open Med, 2021, 9: 20503121211037903. DOI: 10.1177/20503121211037903.
|
| [79] |
WATSON N F, BENCA R M, KRYSTAL A D, et al. Alliance for sleep clinical practice guideline on switching or deprescribing hypnotic medications for insomnia[J]. J Clin Med, 2023, 12(7): 2493. DOI: 10.3390/jcm12072493.
|
| [80] |
TAKAESU Y, SUZUKI M, MOLINE M, et al. Effect of discontinuation of lemborexant following long-term treatment of insomnia disorder: secondary analysis of a randomized clinical trial[J]. Clin Transl Sci, 2023, 16(4): 581-592. DOI: 10.1111/cts.13470.
|
| [81] |
MAYLEBEN D, ROSENBERG R, PINNER K, et al. Assessment of morning sleep propensity with lemborexant in adults with insomnia disorder in a randomized, placebo-controlled crossover study[J]. Sleep Adv, 2021, 2(1): zpab011. DOI: 10.1093/sleepadvances/zpab011.
|
| [82] |
MURPHY P, KUMAR D, ZAMMIT G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening[J]. J Clin Sleep Med, 2020, 16(5): 765-773. DOI: 10.5664/jcsm.8294.
|
| [83] |
VERMEEREN A, JONGEN S, MURPHY P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers[J]. Sleep, 2019, 42(4): zsy260. DOI: 10.1093/sleep/zsy260.
|
| [84] |
VERMEEREN A, VUURMAN E F P M, LEUFKENS T R M, et al. Residual effects of low-dose sublingual zolpidem on highway driving performance the morning after middle-of-the-night use[J]. Sleep, 2014, 37(3): 489-496. DOI: 10.5665/sleep.3482.
|
| [85] |
FORNARO M, CAIAZZA C, ROSSANO F, et al. Residual effects of medications for sleep disorders on driving performance: a systematic review and network meta-analysis of randomized controlled trials: NMA driving and hypnotics[J]. Eur Neuropsychopharmacol, 2024, 81: 53-63. DOI: 10.1016/j.euroneuro.2024.01.011.
|
| [86] |
GOTTER A L, WINROW C J, BRUNNER J, et al. The duration of sleep promoting efficacy by dual orexin receptor antagonists is dependent upon receptor occupancy threshold[J]. BMC Neurosci, 2013, 14: 90. DOI: 10.1186/1471-2202-14-90.
|
| [87] |
NA H J, JEON N, STAATZ C E, et al. Clinical safety and narcolepsy-like symptoms of dual orexin receptor antagonists in patients with insomnia: a systematic review and meta-analysis[J]. Sleep, 2024, 47(2): zsad293. DOI: 10.1093/sleep/zsad293.
|
| [88] |
MCINTYRE R S, WONG S, KWAN A T H, et al. Association between dual orexin receptor antagonists (DORAs) and suicidality: reports to the United States Food and Drug Administration Adverse Event Reporting System (FAERS)[J]. Expert Opin Drug Saf, 2025, 24(6): 753-757. DOI: 10.1080/14740338.2024.2361300.
|
| [89] |
ASAKURA S, SHIOTANI M, GAUVIN D V, et al. Nonclinical evaluation of abuse liability of the dual orexin receptor antagonist lemborexant[J]. Regul Toxicol Pharmacol, 2021, 127: 105053. DOI: 10.1016/j.yrtph.2021.105053.
|
| [90] |
MOLINE M, ASAKURA S, BEUCKMAN C, et al. The abuse potential of lemborexant, a dual orexin receptor antagonist, according to the 8 factors of the Controlled Substances Act[J]. Psychopharmacology, 2023, 240(4): 699-711. DOI: 10.1007/s00213-023-06320-y.
|
| [91] |
MISHIMA K, FUJIMOTO K, ENDO A, et al. Safety and efficacy of lemborexant in insomnia patients: results of a postmarketing observational study of dayvigo® tablets[J]. Drugs R D, 2024, 24(2): 211-226. DOI: 10.1007/s40268-024-00462-w.
|
| [92] |
DAYAL S, ALURI J, HALL N, et al. Effect of hepatic impairment on pharmacokinetics, safety, and tolerability of lemborexant[J]. Pharmacol Res Perspect, 2021, 9(2): e00758. DOI: 10.1002/prp2.758.
|
| [93] |
LANDRY I, ALURI J, HALL N, et al. Effect of severe renal impairment on pharmacokinetics, safety, and tolerability of lemborexant[J]. Pharmacol Res Perspect, 2021, 9(2): e00734. DOI: 10.1002/prp2.734.
|
| [94] |
SAITO J, ISHII M, SANDAIJI N, et al. Lemborexant levels in maternal serum, cord blood, and breast milk during pregnancy and lactation: a case report[J]. PCN Rep, 2023, 2(1): e62. DOI: 10.1002/pcn5.62.
|
| [95] |
RAWAL S, BRIMHALL D, ALURI J, et al. Lemborexant levels in breast milk after single doses in healthy, lactating women[J]. Br J Clin Pharmacol, 2024, 90(1): 158-163. DOI: 10.1111/bcp.15880.
|
| [96] |
GOTFRIED M H, AUERBACH S H, DANG-VU T T, et al. Efficacy and safety of insomnia treatment with lemborexant in older adults: analyses from three clinical trials[J]. Drugs Aging, 2024, 41(9): 741-752. DOI: 10.1007/s40266-024-01135-8.
|
 ), 李亦蕾2,*(
), 李亦蕾2,*( )
)
 ), LI Yilei2,*(
), LI Yilei2,*( )
)