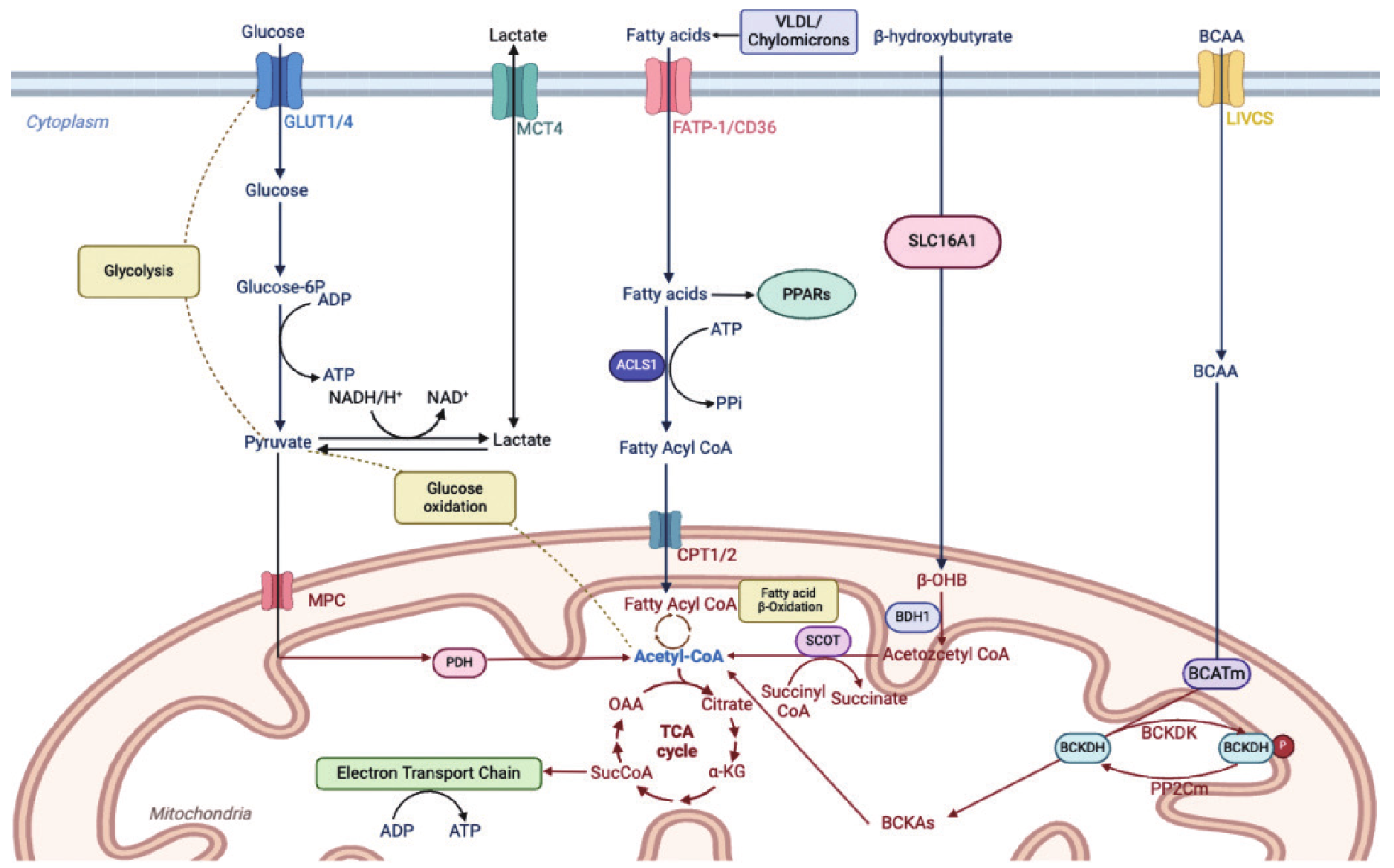
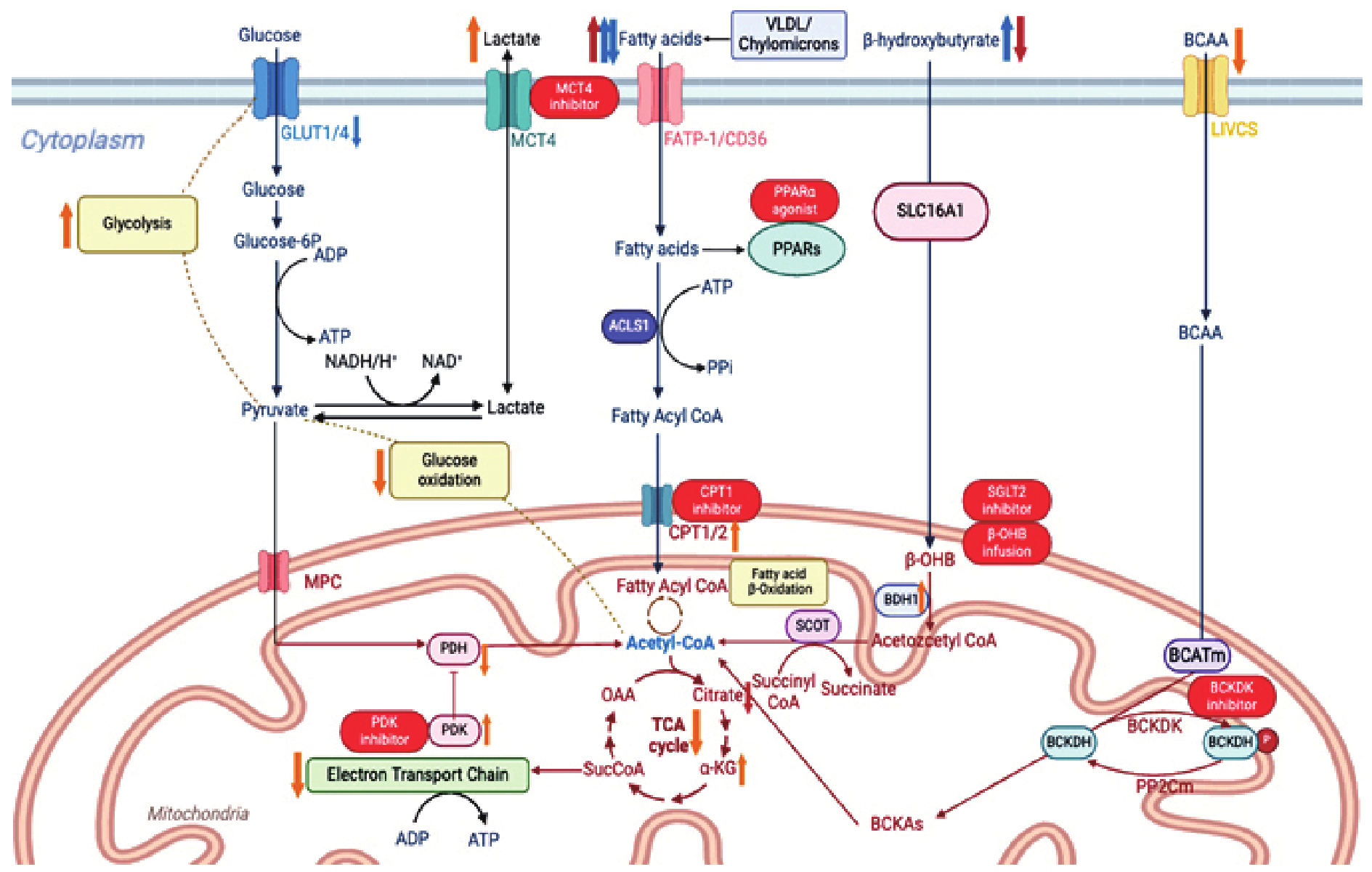
| [34] |
DUNG NGUYEN T, SHINGU Y, AMORIM P A,et al. GLP-1 improves diastolic function and survival in heart failure with preserved ejection fraction[J]. J Cardiovasc Transl Res, 2018, 11(3):259-267. DOI: 10.1007/s12265-018-9795-z.
|
| [35] |
WANG C S, LI L, LIU S Y,et al. GLP-1 receptor agonist ameliorates obesity-induced chronic kidney injury via restoring renal metabolism homeostasis[J]. PLoS One, 2018, 13(3):e0193473. DOI: 10.1371/journal.pone.0193473.
|
| [36] |
KOSIBOROD M N, ABILDSTRØM S Z, BORLAUG B A,et al. Design and baseline characteristics of STEP-HFpEF program evaluating semaglutide in patients with obesity HFpEF phenotype[J]. JACC Heart Fail, 2023, 11(8 Pt 1):1000-1010. DOI: 10.1016/j.jchf.2023.05.010.
|
| [37] |
RYAN D H, LINGVAY I, COLHOUN H M,et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity(SELECT)rationale and design[J]. Am Heart J, 2020, 229:61-69. DOI: 10.1016/j.ahj.2020.07.008.
|
| [38] |
SATTAR N, LEE M M Y, KRISTENSEN S L,et al. Cardiovascular,mortality,and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes:a systematic review and meta-analysis of randomised trials[J]. Lancet Diabetes Endocrinol, 2021, 9(10):653-662. DOI: 10.1016/S2213-8587(21)00203-5.
|
| [39] |
UMBARAWAN Y, SYAMSUNARNO M R A A, KOITABASHI N,et al. Myocardial fatty acid uptake through CD36 is indispensable for sufficient bioenergetic metabolism to prevent progression of pressure overload-induced heart failure[J]. Sci Rep, 2018, 8(1):12035. DOI: 10.1038/s41598-018-30616-1.
|
| [40] |
ZHANG X X, WANG N, FU P,et al. Dapagliflozin attenuates heart failure with preserved ejection fraction remodeling and dysfunction by elevating β-hydroxybutyrate-activated citrate synthase[J]. J Cardiovasc Pharmacol, 2023, 82(5):375-388. DOI: 10.1097/FJC.0000000000001474.
|
| [41] |
AN D Q, ZENG Q C, ZHANG P J,et al. Alpha-ketoglutarate ameliorates pressure overload-induced chronic cardiac dysfunction in mice[J]. Redox Biol, 2021, 46:102088. DOI: 10.1016/j.redox.2021.102088.
|
| [42] |
ALEXANDER T, FISMAN ENRIQUE Z. Impaired glucose metabolism in patients with heart failure:pathophysiology and possible treatment strategies[J]. Am J Cardiovasc Drugs Drugs Devices Other Interv,2004,4(5):269-280.
|
| [43] |
CLUNTUN A A, BADOLIA R, LETTLOVA S,et al. The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure[J]. Cell Metab, 2021, 33(3):629-648.e10. DOI: 10.1016/j.cmet.2020.12.003.
|
| [44] |
CLODI M, RESL M, STELZENEDER D,et al. Interactions of glucose metabolism and chronic heart failure[J]. Exp Clin Endocrinol Diabetes, 2009, 117(3):99-106. DOI: 10.1055/s-2008-1081211.
|
| [45] |
DÁVILA-ROMÁN V G, VEDALA G, HERRERO P,et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy[J]. J Am Coll Cardiol, 2002, 40(2):271-277. DOI: 10.1016/s0735-1097(02)01967-8.
|
| [46] |
ROSENBLATT-VELIN N, MONTESSUIT C, PAPAGEORGIOU I,et al. Postinfarction heart failure in rats is associated with upregulation of GLUT-1 and downregulation of genes of fatty acid metabolism[J]. Cardiovasc Res, 2001, 52(3):407-416. DOI: 10.1016/s0008-6363(01)00393-5.
|
| [47] |
LEE S H, HADIPOUR-LAKMEHSARI S, KIM D H,et al. Bioinformatic analysis of membrane and associated proteins in murine cardiomyocytes and human myocardium[J]. Sci Data, 2020, 7(1):425. DOI: 10.1038/s41597-020-00762-1.
|
| [48] |
JUN M R, BASU R, MCLEAN B A,et al. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation:a metabolic contribution to heart failure with normal ejection fraction[J]. Circ Heart Fail, 2012, 5(4):493-503. DOI: 10.1161/CIRCHEARTFAILURE.112.966705.
|
| [49] |
CHRISTE M E, RODGERS R L. Cardiac glucose and fatty acid oxidation in the streptozotocin-induced diabetic spontaneously hypertensive rat[J]. Hypertension, 1995, 25(2):235-241. DOI: 10.1161/01.hyp.25.2.235.
|
| [50] |
SANKARALINGAM S, ABO ALROB O, ZHANG L Y,et al. Lowering body weight in obese mice with diastolic heart failure improves cardiac insulin sensitivity and function:implications for the obesity paradox[J]. Diabetes, 2015, 64(5):1643-1657. DOI: 10.2337/db14-1050.
|
| [51] |
ZHANG L Y, JASWAL J S, USSHER J R,et al. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy[J]. Circ Heart Fail, 2013, 6(5):1039-1048. DOI: 10.1161/CIRCHEARTFAILURE.112.000228.
|
| [1] |
BOZKURT B, COATS A J S, TSUTSUI H,et al. Universal definition and classification of heart failure:a report of the Heart Failure Society of America,Heart Failure Association of the European Society of Cardiology,Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure:Endorsed by the Canadian Heart Failure Society,Heart Failure Association of India,Cardiac Society of Australia and New Zealand,and Chinese Heart Failure Association[J]. Eur J Heart Fail, 2021, 23(3):352-380. DOI: 10.1002/ejhf.2115.
|
| [2] |
DUNLAY S M, ROGER V L, REDFIELD M M. Epidemiology of heart failure with preserved ejection fraction[J]. Nat Rev Cardiol, 2017, 14(10):591-602. DOI: 10.1038/nrcardio.2017.65.
|
| [3] |
LOPASCHUK G D, DYCK J R B. Ketones and the cardiovascular system[J]. Nat Cardiovasc Res, 2023, 2(5):425-437. DOI: 10.1038/s44161-023-00259-1.
|
| [4] |
KARWI Q G, UDDIN G M, HO K L,et al. Loss of metabolic flexibility in the failing heart[J]. Front Cardiovasc Med, 2018, 5:68. DOI: 10.3389/fcvm.2018.00068.
|
| [5] |
SADDIK M, LOPASCHUK G D. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts[J]. J Biol Chem,1991,266(13):8162-8170.
|
| [6] |
TUOMAINEN T, TAVI P. The role of cardiac energy metabolism in cardiac hypertrophy and failure[J]. Exp Cell Res, 2017, 360(1):12-18. DOI: 10.1016/j.yexcr.2017.03.052.
|
| [7] |
SUN Q Y, KARWI Q G, WONG N,et al. Advances in myocardial energy metabolism:metabolic remodelling in heart failure and beyond[J]. Cardiovasc Res, 2024, 120(16):1996-2016. DOI: 10.1093/cvr/cvae231.
|
| [8] |
KOLWICZ S C Jr, PUROHIT S, TIAN R. Cardiac metabolism and its interactions with contraction,growth,and survival of cardiomyocytes[J]. Circ Res, 2013, 113(5):603-616. DOI: 10.1161/CIRCRESAHA.113.302095.
|
| [9] |
LOPASCHUK G D, KARWI Q G, TIAN R,et al. Cardiac energy metabolism in heart failure[J]. Circ Res, 2021, 128(10):1487-1513. DOI: 10.1161/CIRCRESAHA.121.318241.
|
| [52] |
SUN Q Y, WAGG C S, GÜVEN B,et al. Stimulating cardiac glucose oxidation lessens the severity of heart failure in aged female mice[J]. Basic Res Cardiol, 2024, 119(1):133-150. DOI: 10.1007/s00395-023-01020-2.
|
| [53] |
GROVER-MCKAY M, SCHWAIGER M, KRIVOKAPICH J,et al. Regional myocardial blood flow and metabolism at rest in mildly symptomatic patients with hypertrophic cardiomyopathy[J]. J Am Coll Cardiol, 1989, 13(2):317-324. DOI: 10.1016/0735-1097(89)90505-6.
|
| [54] |
KATO T, NIIZUMA S, INUZUKA Y,et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure[J]. Circ Heart Fail, 2010, 3(3):420-430. DOI: 10.1161/CIRCHEARTFAILURE.109.888479.
|
| [55] |
TUUNANEN H, ENGBLOM E, NAUM A,et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure[J]. Circulation, 2006, 114(20):2130-2137. DOI: 10.1161/CIRCULATIONAHA.106.645184.
|
| [56] |
NEGLIA D, DE CATERINA A, MARRACCINI P,et al. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy[J]. Am J Physiol Heart Circ Physiol, 2007, 293(6):H3270-3278. DOI: 10.1152/ajpheart.00887.2007.
|
| [57] |
TAYLOR M, WALLHAUS T R, DEGRADO T R,et al. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F] fluoro-6-Thia-heptadecanoic acid and [18F] FDG in Patients with Congestive Heart Failure[J]. J Nucl Med,2001,42(1):55-62.
|
| [58] |
VOROS G, ECTOR J, GARWEG C,et al. Abstract 14 631:increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling[J]. Circ Heart Fail, 2018, 11(12):e004953. DOI: 10.1161/CIRCHEARTFAILURE.118.004953.
|
| [59] |
BAROUCH L A, BERKOWITZ D E, HARRISON R W,et al. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice[J]. Circulation, 2003, 108(6):754-759. DOI: 10.1161/01.CIR.0000083716.82622.FD.
|
| [60] |
DENG Y, XIE M, LI Q,et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF[J]. Circ Res, 2021, 128(2):232-245. DOI: 10.1161/circresaha.120.317933.
|
| [10] |
ABEL E D. Glucose transport in the heart [J]. Front Biosci, 2004, 9:201. DOI: 10.2741/1216.
|
| [11] |
HERZIG S, RAEMY E, MONTESSUIT S,et al. Identification and functional expression of the mitochondrial pyruvate carrier[J]. Science, 2012, 337(6090):93-96. DOI: 10.1126/science.1218530.
|
| [12] |
HOLNESS M J, SUGDEN M C. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation[J]. Biochem Soc Trans, 2003, 31(Pt 6):1143-1151. DOI: 10.1042/bst0311143.
|
| [13] |
DE JONG K A, LOPASCHUK G D. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction[J]. Can J Cardiol, 2017, 33(7):860-871. DOI: 10.1016/j.cjca.2017.03.009.
|
| [14] |
MURTHY M S, PANDE S V. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane[J]. Proc Natl Acad Sci USA, 1987, 84(2):378-382. DOI: 10.1073/pnas.84.2.378.
|
| [15] |
MONTAIGNE D, BUTRUILLE L, STAELS B. PPAR control of metabolism and cardiovascular functions[J]. Nat Rev Cardiol, 2021, 18(12):809-823. DOI: 10.1038/s41569-021-00569-6.
|
| [16] |
HO K L, KARWI Q G, WANG F Q,et al. The ketogenic diet does not improve cardiac function and blunts glucose oxidation in ischaemic heart failure[J]. Cardiovasc Res, 2024, 120(10):1126-1137. DOI: 10.1093/cvr/cvae092.
|
| [17] |
HO K L, ZHANG L Y, WAGG C,et al. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency[J]. Cardiovasc Res, 2019, 115(11):1606-1616. DOI: 10.1093/cvr/cvz045.
|
| [18] |
HO K L, KARWI Q G, WAGG C,et al. Ketones can become the major fuel source for the heart but do not increase cardiac efficiency[J]. Cardiovasc Res, 2021, 117(4):1178-1187. DOI: 10.1093/cvr/cvaa143.
|
| [19] |
SWEATT A J, WOOD M, SURYAWAN A,et al. Branched-chain amino acid catabolism:unique segregation of pathway enzymes in organ systems and peripheral nerves[J]. Am J Physiol Endocrinol Metab, 2004, 286(1):E64-76. DOI: 10.1152/ajpendo.00276.2003.
|
| [61] |
ZHU N, JIANG W B, WANG Y,et al. Plasma levels of free fatty acid differ in patients with left ventricular preserved,mid-range,and reduced ejection fraction[J]. BMC Cardiovasc Disord, 2018, 18(1):104. DOI: 10.1186/s12872-018-0850-0.
|
| [62] |
MURASHIGE D, JANG C, NEINAST M,et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart[J]. Science, 2020, 370(6514):364-368. DOI: 10.1126/science.abc8861.
|
| [63] |
FENG J H, ZHAO H, DU M Z,et al. The effect of apelin-13 on pancreatic islet beta cell mass and myocardial fatty acid and glucose metabolism of experimental type 2 diabetic rats[J]. Peptides, 2019, 114:1-7. DOI: 10.1016/j.peptides.2019.03.006.
|
| [64] |
CHEN X, WANG Q Y, SHAO M Y,et al. Ginsenoside Rb3 regulates energy metabolism and apoptosis in cardiomyocytes via activating PPARα pathway[J]. Biomed Pharmacother, 2019, 120:109487. DOI: 10.1016/j.biopha.2019.109487.
|
| [65] |
KOLLERITSCH S, KIEN B, SCHOISWOHL G,et al. Low cardiac lipolysis reduces mitochondrial fission and prevents lipotoxic heart dysfunction in Perilipin 5 mutant mice[J]. Cardiovasc Res, 2020, 116(2):339-352. DOI: 10.1093/cvr/cvz119.
|
| [66] |
SHU H Y, HANG W J, PENG Y Z,et al. Trimetazidine attenuates heart failure by improving myocardial metabolism via AMPK[J]. Front Pharmacol, 2021, 12:707399. DOI: 10.3389/fphar.2021.707399.
|
| [67] |
MARZILLI M, VINEREANU D, LOPASCHUK G,et al. Trimetazidine in cardiovascular medicine[J]. Int J Cardiol, 2019, 293:39-44. DOI: 10.1016/j.ijcard.2019.05.063.
|
| [68] |
KHUCHUA Z, GLUKHOV A I, STRAUSS A W,et al. Elucidating the beneficial role of PPAR agonists in cardiac diseases[J]. Int J Mol Sci, 2018, 19(11):3464. DOI: 10.3390/ijms19113464.
|
| [69] |
NIELSEN R, MØLLER N, GORMSEN L C,et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients[J]. Circulation, 2019, 139(18):2129-2141. DOI: 10.1161/CIRCULATIONAHA.118.036459.
|
| [20] |
KARWI Q G, LOPASCHUK G D. Branched-chain amino acid metabolism in the failing heart[J]. Cardiovasc Drugs Ther, 2023, 37(2):413-420. DOI: 10.1007/s10557-022-07320-4.
|
| [21] |
KARWI Q G, ZHANG L Y, WAGG C S,et al. Targeting the glucagon receptor improves cardiac function and enhances insulin sensitivity following a myocardial infarction[J]. Cardiovasc Diabetol, 2019, 18(1):1. DOI: 10.1186/s12933-019-0806-4.
|
| [22] |
HALLAJ NEISHABOURI S, HUTSON S M, DAVOODI J. Chronic activation of mTOR complex 1 by branched chain amino acids and organ hypertrophy[J]. Amino Acids, 2015, 47(6):1167-1182. DOI: 10.1007/s00726-015-1944-y.
|
| [23] |
SHAO D, VILLET O, ZHANG Z,et al. Glucose promotes cell growth by suppressing branched-chain amino acid degradation[J]. Nat Commun, 2018, 9(1):2935. DOI: 10.1038/s41467-018-05362-7.
|
| [24] |
WANG T J, LARSON M G, VASAN R S,et al. Metabolite profiles and the risk of developing diabetes[J]. Nat Med, 2011, 17:448-453. DOI: 10.1038/nm.2307.
|
| [25] |
LOPASCHUK G D, KARWI Q G, TIAN R,et al. Cardiac energy metabolism in heart failure[J]. Circ Res, 2021, 128(10):1487-1513. DOI: 10.1161/CIRCRESAHA.121.318241.
|
| [26] |
SONG M S, MIHARA K, CHEN Y,et al. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts[J]. Cell Metab, 2015, 21(2):273-286. DOI: 10.1016/j.cmet.2014.12.011.
|
| [27] |
|
| [28] |
DORN G W 2nd, VEGA R B, KELLY D P,et al. Mitochondrial biogenesis and dynamics in the developing and diseased heart [J]. Genes Dev, 2015, 29(19):1981-1891. DOI: 10.1101/gad.269894.115.
|
| [70] |
HORTON J L, DAVIDSON M T, KURISHIMA C,et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense[J]. JCI Insight, 2019, 4(4):e124079. DOI: 10.1172/jci.insight.124079.
|
| [71] |
VOROS G, ECTOR J, GARWEG C,et al. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling[J]. Circ Heart Fail, 2018, 11(12):e004953. DOI: 10.1161/CIRCHEARTFAILURE.118.004953.
|
| [72] |
ZORDOKY B N, SUNG M M, EZEKOWITZ J,et al. Metabolomic fingerprint of heart failure with preserved ejection fraction[J]. PLoS One, 2015, 10(5):e0124844. DOI: 10.1371/journal.pone.0124844.
|
| [73] |
ANKER S D, BUTLER J, FILIPPATOS G,et al. Empagliflozin in heart failure with a preserved ejection fraction[J]. N Engl J Med, 2021, 385(16):1451-1461. DOI: 10.1056/NEJMoa2107038.
|
| [74] |
PETERSON M B, MEAD R J, WELTY J D. Free amino acids in congestive heart failure[J]. J Mol Cell Cardiol, 1973, 5(2):139-147. DOI: 10.1016/0022-2828(73)90047-3.
|
| [75] |
LAI L, LEONE T C, KELLER M P,et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart:a multisystems approach[J]. Circ Heart Fail, 2014, 7(6):1022-1031. DOI: 10.1161/CIRCHEARTFAILURE.114.001469.
|
| [76] |
SUN H P, OLSON K C, GAO C,et al. Catabolic defect of branched-chain amino acids promotes heart failure[J]. Circulation, 2016, 133(21):2038-2049. DOI: 10.1161/CIRCULATIONAHA.115.020226.
|
| [77] |
UDDIN G M, ZHANG L Y, SHAH S,et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure[J]. Cardiovasc Diabetol, 2019, 18(1):86. DOI: 10.1186/s12933-019-0892-3.
|
| [78] |
HAHN V S, PETUCCI C, KIM M S,et al. Myocardial metabolomics of human heart failure with preserved ejection fraction[J]. Circulation, 2023, 147(15):1147-1161. DOI: 10.1161/CIRCULATIONAHA.122.061846.
|
| [29] |
INGWALL J S, WEISS R G. Is the failing heart energy starved? On using chemical energy to support cardiac function[J]. Circ Res, 2004, 95(2):135-145. DOI: 10.1161/01.RES.0000137170.41939.d9.
|
| [30] |
DYCK J R B, SOSSALLA S, HAMDANI N,et al. Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure:evidence for potential off-target effects[J]. J Mol Cell Cardiol, 2022, 167:17-31. DOI: 10.1016/j.yjmcc.2022.03.005.
|
| [31] |
CAPONE F, NAMBIAR N, SCHIATTARELLA G G. Beyond weight loss:the emerging role of incretin-based treatments in cardiometabolic HFpEF[J]. Curr Opin Cardiol, 2024, 39(3):148-153. DOI: 10.1097/HCO.0000000000001117.
|
| [32] |
FERRANNINI E, BALDI S, FRASCERRA S,et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes[J]. Diabetes, 2016, 65(5):1190-1195. DOI: 10.2337/db15-1356.
|
| [33] |
CHAUDHURI A, GHANIM H, VORA M,et al. Exenatide exerts a potent antiinflammatory effect[J]. J Clin Endocrinol Metab, 2012, 97(1):198-207. DOI: 10.1210/jc.2011-1508.
|
| [79] |
ZHANG Z Y, FENG X Y, WANG Z H,et al. Similarities and differences of myocardial metabolic characteristics between HFpEF and HFrEF mice based on LC-MS/MS metabolomics[J]. Zhonghua Xin Xue Guan Bing Za Zhi, 2023, 51(7):722-730. DOI: 10.3760/cma.j.cn112148-20230329-00182.
|
| [80] |
NOORDALI H, LOUDON B L, FRENNEAUX M P,et al. Cardiac metabolism—a promising therapeutic target for heart failure[J]. Pharmacol Ther, 2018, 182:95-114. DOI: 10.1016/j.pharmthera.2017.08.001.
|
| [81] |
FRAGASSO G, PALLOSHI A, PUCCETTI P,et al. A randomized clinical trial of trimetazidine,a partial free fatty acid oxidation inhibitor,in patients with heart failure[J]. J Am Coll Cardiol, 2006, 48(5):992-998. DOI: 10.1016/j.jacc.2006.03.060.
|
| [82] |
LEE L, CAMPBELL R, SCHEUERMANN-FREESTONE M,et al. Metabolic modulation with perhexiline in chronic heart failure:a randomized,controlled trial of short-term use of a novel treatment[J]. Circulation, 2005, 112(21):3280-3288. DOI: 10.1161/CIRCULATIONAHA.105.551457.
|
| [83] |
BERSIN R M, WOLFE C, KWASMAN M,et al. Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate[J]. J Am Coll Cardiol, 1994, 23(7):1617-1624. DOI: 10.1016/0735-1097(94)90665-3.
|
| [84] |
ZHOU B, TIAN R. Mitochondrial dysfunction in pathophysiology of heart failure[J]. J Clin Invest, 2018, 128(9):3716-3726. DOI: 10.1172/JCI120849.
|
| [85] |
AL SAADI T, ASSAF Y, FARWATI M,et al. Coenzyme Q10 for heart failure[J]. Cochrane Database Syst Rev, 2021(2):CD008684. DOI: 10.1002/14651858.CD008684.pub3.
|
| [86] |
BUTLER J, KHAN M S, ANKER S D,et al. Effects of elamipretide on left ventricular function in patients with heart failure with reduced ejection fraction:the PROGRESS-HF phase 2 trial[J]. J Card Fail, 2020, 26(5):429-437. DOI: 10.1016/j.cardfail.2020.02.001.
|
| [87] |
LOPASCHUK G D, KARWI Q G, HO K L,et al. Ketone metabolism in the failing heart[J]. Biochim Biophys Acta Mol Cell Biol Lipids, 2020, 1865(12):158813. DOI: 10.1016/j.bbalip.2020.158813.
|
| [88] |
CALIFF R M, O´CONNOR C M. Beta-blocker therapy for heart failure:the evidence is in,now the work begins[J]. JAMA, 2000, 283(10):1335-1337. DOI: 10.1001/jama.283.10.1335.
|
| [89] |
PARODI-RULLÁN R M, CHAPA-DUBOCQ X, GUZMÁN-HERNÁNDEZ R,et al. The role of adenine nucleotide translocase in the assembly of respiratory super complexes in cardiac cells[J]. Cells, 2019, 8(10):1247. DOI: 10.3390/cells8101247.
|
| [90] |
OKA S I, SABRY A D, CAWLEY K M,et al. Multiple levels of PGC-1α dysregulation in heart failure[J]. Front Cardiovasc Med, 2020, 7:2. DOI: 10.3389/fcvm.2020.00002.
|
| [91] |
|
 )
)