中国全科医学 ›› 2025, Vol. 28 ›› Issue (23): 2861-2869.DOI: 10.12114/j.issn.1007-9572.2024.0527
吴莎1, 张代义2, 李晋1, 宣勤考3, 钱晓东3, 朱传武1, 浦剑虹2, 朱莉1,*( )
)
收稿日期:2024-11-19
修回日期:2025-02-16
出版日期:2025-08-15
发布日期:2025-06-17
通讯作者:
朱莉
吴莎和张代义共同为第一作者
作者贡献:
吴莎负责数据整理分析、论文撰写及修改;张代义负责数据整理及分析;李晋负责数据分析和论文修改;宣勤考、钱晓东负责数据收集;朱传武、浦剑虹负责研究思路指导;朱莉负责研究设计构思、研究思路指导、数据整体分析、论文修改指导。
基金资助:
WU Sha1, ZHANG Daiyi2, LI Jin1, XUAN Qinkao3, QIAN Xiaodong3, ZHU Chuanwu1, PU Jianhong2, ZHU Li1,*( )
)
Received:2024-11-19
Revised:2025-02-16
Published:2025-08-15
Online:2025-06-17
Contact:
ZHU Li
About author:WU Sha and ZHANG Daiyi are co-first authors
摘要: 背景 代谢相关脂肪性肝病(MAFLD)与2型糖尿病(T2DM)共存的全球流行率和发生率持续上升,这种共存增加了肝脏相关不良结局的风险。临床实践中需要对MAFLD合并高血糖的高风险患者进行筛查和早期诊断,以延缓其进展。 目的 基于T2DM与MAFLD之间的关系,利用现有大规模体检数据探究高血糖对MAFLD肝脂肪变性和肝纤维化的影响,并分析影响MAFLD合并高血糖发生的关键因素。 方法 收集2024年3—7月苏州大学附属第一医院18 286名健康体检者的基本信息、既往史、腹部超声检查、生化指标和血常规项目等数据。根据腹部超声检查结果及MAFLD诊断标准,筛选出5 258例MAFLD患者,将MAFLD患者根据基于4因子的纤维化指数(FIB-4)水平分为T1组(FIB-4<1.30,n=4 275)、T2组(1.30≤FIB-4≤2.67,n=924)和T3组(FIB-4>2.67,n=59),比较三组间各临床指标差异。进一步根据有无糖尿病史、空腹血糖(FBG)≥7.0 mmol/L、糖化血红蛋白(HbA1c)≥6.5%(满足任1项)将MAFLD患者分为MAFLD合并高糖组(n=752)和MAFLD合并无糖组(n=4 506),比较两组肝脂肪变性和肝纤维化相关指标差异。采用单因素及多因素Logistic回归分析探究影响MAFLD合并高血糖发生的关键因素。采用受试者工作特征(ROC)曲线评估联合预测模型对MAFLD合并高血糖发生的预测价值。 结果 T1组、T2组、T3组吸烟、高血压、糖尿病、高脂血症、高尿酸血症、冠心病、年龄、BMI、FBG、HbA1c、血小板计数(PLT)、白细胞计数(WBC)、红细胞计数(RBC)、血红蛋白(Hb)、红细胞分布宽度(RDW)、中性粒细胞计数(NEUT)、淋巴细胞计数(LYM)、单核细胞计数(MONO)、丙氨酸氨基转移酶(ALT)、天冬氨酸氨基转移酶(AST)、总胆固醇(TC)、三酰甘油(TG)、高密度脂蛋白胆固醇(HDL-C)、低密度脂蛋白胆固醇(LDL-C)、白蛋白(ALB)、γ-谷氨酰转肽酶(GGT)、尿酸(UA)、肌酐(Cr)、尿素氮(BUN)、总胆红素(TBIL)、直接胆红素(DBIL)、间接胆红素(IBIL)、碱性磷酸酶(ALP)、预估葡萄糖处理率(eGDR)比较,差异均有统计学意义(P<0.05)。MAFLD合并高糖组脂肪肝指数(FLI)、肝脂肪变性指数(HSI)、ZJU、FIB-4、AST/PLT比值(APRI)、非酒精性脂肪肝纤维化评分(NFS)和BMI、AST/ALT及糖尿病评分(BARD)均高于MAFLD合并无糖组(P<0.05)。将MAFLD合并高糖组和MAFLD合并无糖组分别按1∶1随机纳入训练集和测试集,训练集中所有指标进行单因素及多因素Logistic回归分析,结果显示,年龄、腰围(WC)、高血压、高脂血症、TG、GGT、UA、BUN是MAFLD合并高血糖发生的影响因素(P<0.05)。进一步将以上指标进行ROC曲线分析,结果显示年龄、WC、高血压、高脂血症、TG、GGT、UA、BUN作为独立预测因子判断MAFLD合并高血糖发生具有一定的预测准确度[0.53≤ROC曲线下面积(AUC)≤0.75]。联合这8项关键预测因子构建预测模型并绘制ROC曲线,结果显示,联合模型预测准确度达到0.805(95%CI=0.781~0.828),灵敏度为75.8%,特异度为72.6%。在测试集中对该联合模型进行效能验证,结果显示阳性预测值为70.5%,阴性预测值为73.1%,预测准确率为72.7%。 结论 基于FIB-4分组的MAFLD患者,高血压、FBG、HbA1c、PLT、WBC、RBC、LYM、AST、eGDR水平在3组间有显著差异。机体高血糖能够加重MAFLD的肝脂肪变性和肝纤维化程度。此外,年龄、WC、高血压、高脂血症、TG、GGT、UA、BUN是导致MAFLD向MAFLD合并高血糖进展的影响因素。联合上述8项指标构建预测模型能够增强MAFLD人群合并高血糖发生的预测准确率,可能为临床上MAFLD人群合并高血糖的早期鉴别提供参考依据。
中图分类号:
| 组别 | 例数 | 性别(男/女) | 吸烟[例(%)] | 饮酒(无/偶尔/适量) | 高血压[例(%)] | 糖尿病[例(%)] | 高脂血症[例(%)] | 高尿酸血症[例(%)] | 冠心病[例(%)] | 年龄[M(P25,P75),岁] | BMI[M(P25,P75),kg/m2] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1组 | 4 275 | 3 151/1 124 | 1 015(23.7) | 3 443/746/86 | 845(19.8) | 245(5.7) | 2 516(58.9) | 1 537(36.0) | 12(0.3) | 43(36,52) | 26.4(24.6,28.6) |
| T2组 | 924 | 670/254 | 267(28.9) | 750/152/22 | 430(46.5) | 154(16.7) | 492(53.2) | 270(29.2) | 5(0.5) | 62(58,70) | 25.9(24.3,28.0) |
| T3组 | 59 | 40/19 | 11(18.6) | 47/11/1 | 26(44.1) | 11(18.6) | 29(49.2) | 24(40.7) | 2(3.4) | 75(65,79) | 25.9(24.0,28.4) |
| H(χ2)值 | 1.529a | 12.019a | 1.088a | 304.386a | 136.143a | 11.602a | 16.074a | 16.631a | 1 607.011 | 22.324 | |
| P值 | 0.466 | 0.002 | 0.896 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 组别 | WC[M(P25,P75),cm] | FBG [M(P25,P75),mmol/L] | HbA1c[M(P25,P75),%] | PLT[M(P25,P75),×109/L] | WBC[M(P25,P75),×109/L] | RBC[M(P25,P75),×1012/L] | Hb[M(P25,P75),g/L] | RDW[M(P25,P75),%] | NEUT [M(P25,P75),×109/L] | LYM [M(P25,P75),×109/L] | MONO [M(P25,P75),×109/L] |
| T1组 | 87 (82,93) | 5.1 (4.8,5.6) | 5.5 (5.2,5.8) | 248 (217,284) | 6.5 (5.6,7.6) | 5.0 (4.7,5.3) | 151 (141,158) | 12.4 (12.1,12.9) | 3.6 (2.9,4.3) | 2.2 (1.9,2.6) | 0.5 (0.4,0.6) |
| T2组 | 88 (82,93) | 5.5 (5.0,6.4) | 5.8 (5.5,6.3) | 187 (164,212) | 5.9 (5.1,6.8) | 4.8 (4.5,5.1) | 147 (139,156) | 12.5 (12.1,13.0) | 3.2 (2.7,3.9) | 2.0 (1.7,2.3) | 0.4 (0.4,0.5) |
| T3组 | 89 (83,93) | 5.5 (5.1,6.5) | 5.7 (5.5,6.3) | 134 (115,153) | 5.7 (4.4,6.8) | 4.5 (4.1,4.9) | 142 (130,154) | 12.9 (12.4,13.3) | 3.1 (2.4,4.2) | 1.8 (1.4,2.2) | 0.4 (0.4,0.5) |
| H(χ2)值 | 2.361 | 167.310 | 276.315 | 1 266.560 | 147.749 | 171.596 | 41.318 | 25.071 | 90.600 | 158.419 | 21.558 |
| P值 | 0.307 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 组别 | NEUT%[M(P25,P75),%] | ALT[M(P25,P75),U/L] | AST[M(P25,P75),U/L] | TC[M(P25,P75),mmol/L] | TG[M(P25,P75),mmol/L] | HDL-C[M(P25,P75),mmol/L] | LDL-C[M(P25,P75),mmol/L] | ALB[M(P25,P75),g/L] | GLB[M(P25,P75),g/L] | GGT[M(P25,P75),U/L] | |
| T1组 | 55.1 (50.0,60.1) | 27.0 (19.0,40.2) | 20.6 (17.0,25.5) | 5.0 (4.4,5.6) | 1.8 (1.3,2.6) | 1.1 (0.9,1.2) | 3.0 (2.5,3.6) | 44.6 (43.1,46.1) | 29.4 (27.2,31.6) | 30.2 (21.3,45.1) | |
| T2组 | 54.6 (49.7,60.8) | 22.0 (16.8,31.9) | 23.0 (19.2,27.6) | 4.8 (4.1,5.5) | 1.7 (1.3,2.4) | 1.1 (0.9,1.3) | 2.8 (2.2,3.4) | 43.8 (42.3,45.2) | 29.2 (27.2,31.5) | 25.9 (19.1,37.3) | |
| T3组 | 57.2 (49.9,63.4) | 25.2 (17.5,43.3) | 31.6 (23.5,41.9) | 4.6 (4.0,5.2) | 1.6 (1.3,2.2) | 1.1 (1.0,1.2) | 2.6 (2.1,3.2) | 43.4 (41.9,44.2) | 29.9 (27.8,32.9) | 28.0 (20.0,41.0) | |
| H(χ2)值 | 1.567 | 95.083 | 155.934 | 34.473 | 12.372 | 15.858 | 72.331 | 131.916 | 4.397 | 59.953 | |
| P值 | 0.457 | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | 0.111 | <0.001 | |
| 组别 | UA[M(P25,P75),μmol/L] | Cr[M(P25,P75),μmol/L] | BUN[M(P25,P75),mmol/L] | TBIL[M(P25,P75),μmol/L] | DBIL[M(P25,P75),μmol/L] | IBIL[M(P25,P75),μmol/L] | ALP[M(P25,P75),U/L] | TyG[M(P25,P75)] | TyG-BMI[M(P25,P75)] | eGDR[M(P25,P75)] | |
| T1组 | 388 (336,448) | 70.4 (59.0,79.0) | 5.1 (4.4,6.0) | 12.9 (10.2,16.4) | 3.6 (2.7,4.7) | 9.3 (7.3,11.7) | 74.0 (63.0,87.3) | 1.6 (1.2,1.9) | 41.7 (32.1,52.5) | 9.6 (8.3,10.2) | |
| T2组 | 373 (328,431) | 72.0 (61.2,82.4) | 5.6 (4.8,6.6) | 14.4 (11.7,18.0) | 4.1 (3.2,5.3) | 10.3 (8.3,12.9) | 77.8 (65.2,91.5) | 1.6 (1.3,2.0) | 41.5 (31.9,52.7) | 8.6 (6.3,10.0) | |
| T3组 | 384 (328,441) | 74.0 (58.7,86.7) | 5.5 (4.7,6.5) | 14.7 (11.5,20.3) | 4.7 (3.7,6.0) | 9.9 (8.1,14.0) | 77.2 (65.0,101.0) | 1.6 (1.3,2.1) | 37.1 (31.7,56.9) | 7.8 (6.3,10.1) | |
| H(χ2)值 | 18.612 | 20.911 | 126.048 | 83.877 | 90.906 | 68.091 | 20.328 | 0.858 | 0.368 | 162.073 | |
| P值 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.651 | 0.832 | <0.001 |
表1 不同FIB-4水平分组的研究对象一般资料比较
Table 1 Comparison of general data of subjects among groups with different FIB-4 levels
| 组别 | 例数 | 性别(男/女) | 吸烟[例(%)] | 饮酒(无/偶尔/适量) | 高血压[例(%)] | 糖尿病[例(%)] | 高脂血症[例(%)] | 高尿酸血症[例(%)] | 冠心病[例(%)] | 年龄[M(P25,P75),岁] | BMI[M(P25,P75),kg/m2] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1组 | 4 275 | 3 151/1 124 | 1 015(23.7) | 3 443/746/86 | 845(19.8) | 245(5.7) | 2 516(58.9) | 1 537(36.0) | 12(0.3) | 43(36,52) | 26.4(24.6,28.6) |
| T2组 | 924 | 670/254 | 267(28.9) | 750/152/22 | 430(46.5) | 154(16.7) | 492(53.2) | 270(29.2) | 5(0.5) | 62(58,70) | 25.9(24.3,28.0) |
| T3组 | 59 | 40/19 | 11(18.6) | 47/11/1 | 26(44.1) | 11(18.6) | 29(49.2) | 24(40.7) | 2(3.4) | 75(65,79) | 25.9(24.0,28.4) |
| H(χ2)值 | 1.529a | 12.019a | 1.088a | 304.386a | 136.143a | 11.602a | 16.074a | 16.631a | 1 607.011 | 22.324 | |
| P值 | 0.466 | 0.002 | 0.896 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 组别 | WC[M(P25,P75),cm] | FBG [M(P25,P75),mmol/L] | HbA1c[M(P25,P75),%] | PLT[M(P25,P75),×109/L] | WBC[M(P25,P75),×109/L] | RBC[M(P25,P75),×1012/L] | Hb[M(P25,P75),g/L] | RDW[M(P25,P75),%] | NEUT [M(P25,P75),×109/L] | LYM [M(P25,P75),×109/L] | MONO [M(P25,P75),×109/L] |
| T1组 | 87 (82,93) | 5.1 (4.8,5.6) | 5.5 (5.2,5.8) | 248 (217,284) | 6.5 (5.6,7.6) | 5.0 (4.7,5.3) | 151 (141,158) | 12.4 (12.1,12.9) | 3.6 (2.9,4.3) | 2.2 (1.9,2.6) | 0.5 (0.4,0.6) |
| T2组 | 88 (82,93) | 5.5 (5.0,6.4) | 5.8 (5.5,6.3) | 187 (164,212) | 5.9 (5.1,6.8) | 4.8 (4.5,5.1) | 147 (139,156) | 12.5 (12.1,13.0) | 3.2 (2.7,3.9) | 2.0 (1.7,2.3) | 0.4 (0.4,0.5) |
| T3组 | 89 (83,93) | 5.5 (5.1,6.5) | 5.7 (5.5,6.3) | 134 (115,153) | 5.7 (4.4,6.8) | 4.5 (4.1,4.9) | 142 (130,154) | 12.9 (12.4,13.3) | 3.1 (2.4,4.2) | 1.8 (1.4,2.2) | 0.4 (0.4,0.5) |
| H(χ2)值 | 2.361 | 167.310 | 276.315 | 1 266.560 | 147.749 | 171.596 | 41.318 | 25.071 | 90.600 | 158.419 | 21.558 |
| P值 | 0.307 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 组别 | NEUT%[M(P25,P75),%] | ALT[M(P25,P75),U/L] | AST[M(P25,P75),U/L] | TC[M(P25,P75),mmol/L] | TG[M(P25,P75),mmol/L] | HDL-C[M(P25,P75),mmol/L] | LDL-C[M(P25,P75),mmol/L] | ALB[M(P25,P75),g/L] | GLB[M(P25,P75),g/L] | GGT[M(P25,P75),U/L] | |
| T1组 | 55.1 (50.0,60.1) | 27.0 (19.0,40.2) | 20.6 (17.0,25.5) | 5.0 (4.4,5.6) | 1.8 (1.3,2.6) | 1.1 (0.9,1.2) | 3.0 (2.5,3.6) | 44.6 (43.1,46.1) | 29.4 (27.2,31.6) | 30.2 (21.3,45.1) | |
| T2组 | 54.6 (49.7,60.8) | 22.0 (16.8,31.9) | 23.0 (19.2,27.6) | 4.8 (4.1,5.5) | 1.7 (1.3,2.4) | 1.1 (0.9,1.3) | 2.8 (2.2,3.4) | 43.8 (42.3,45.2) | 29.2 (27.2,31.5) | 25.9 (19.1,37.3) | |
| T3组 | 57.2 (49.9,63.4) | 25.2 (17.5,43.3) | 31.6 (23.5,41.9) | 4.6 (4.0,5.2) | 1.6 (1.3,2.2) | 1.1 (1.0,1.2) | 2.6 (2.1,3.2) | 43.4 (41.9,44.2) | 29.9 (27.8,32.9) | 28.0 (20.0,41.0) | |
| H(χ2)值 | 1.567 | 95.083 | 155.934 | 34.473 | 12.372 | 15.858 | 72.331 | 131.916 | 4.397 | 59.953 | |
| P值 | 0.457 | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | 0.111 | <0.001 | |
| 组别 | UA[M(P25,P75),μmol/L] | Cr[M(P25,P75),μmol/L] | BUN[M(P25,P75),mmol/L] | TBIL[M(P25,P75),μmol/L] | DBIL[M(P25,P75),μmol/L] | IBIL[M(P25,P75),μmol/L] | ALP[M(P25,P75),U/L] | TyG[M(P25,P75)] | TyG-BMI[M(P25,P75)] | eGDR[M(P25,P75)] | |
| T1组 | 388 (336,448) | 70.4 (59.0,79.0) | 5.1 (4.4,6.0) | 12.9 (10.2,16.4) | 3.6 (2.7,4.7) | 9.3 (7.3,11.7) | 74.0 (63.0,87.3) | 1.6 (1.2,1.9) | 41.7 (32.1,52.5) | 9.6 (8.3,10.2) | |
| T2组 | 373 (328,431) | 72.0 (61.2,82.4) | 5.6 (4.8,6.6) | 14.4 (11.7,18.0) | 4.1 (3.2,5.3) | 10.3 (8.3,12.9) | 77.8 (65.2,91.5) | 1.6 (1.3,2.0) | 41.5 (31.9,52.7) | 8.6 (6.3,10.0) | |
| T3组 | 384 (328,441) | 74.0 (58.7,86.7) | 5.5 (4.7,6.5) | 14.7 (11.5,20.3) | 4.7 (3.7,6.0) | 9.9 (8.1,14.0) | 77.2 (65.0,101.0) | 1.6 (1.3,2.1) | 37.1 (31.7,56.9) | 7.8 (6.3,10.1) | |
| H(χ2)值 | 18.612 | 20.911 | 126.048 | 83.877 | 90.906 | 68.091 | 20.328 | 0.858 | 0.368 | 162.073 | |
| P值 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.651 | 0.832 | <0.001 |
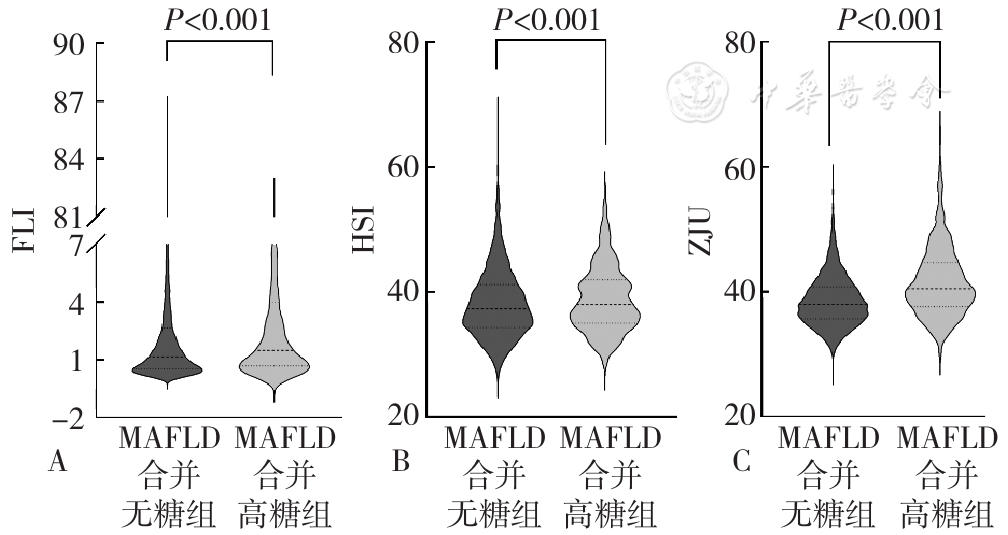
图1 MAFLD合并高糖组与MAFLD合并无糖组肝脂肪变性相关指数比较注:A为脂肪肝指数(FLI),B为肝脂肪变性指数(HSI),C为ZJU指数;MAFLD=代谢相关脂肪性肝病。
Figure 1 Comparison of liver steatosis related indices between MAFLD with hyperglycemia group and MAFLD without hyperglycemia group
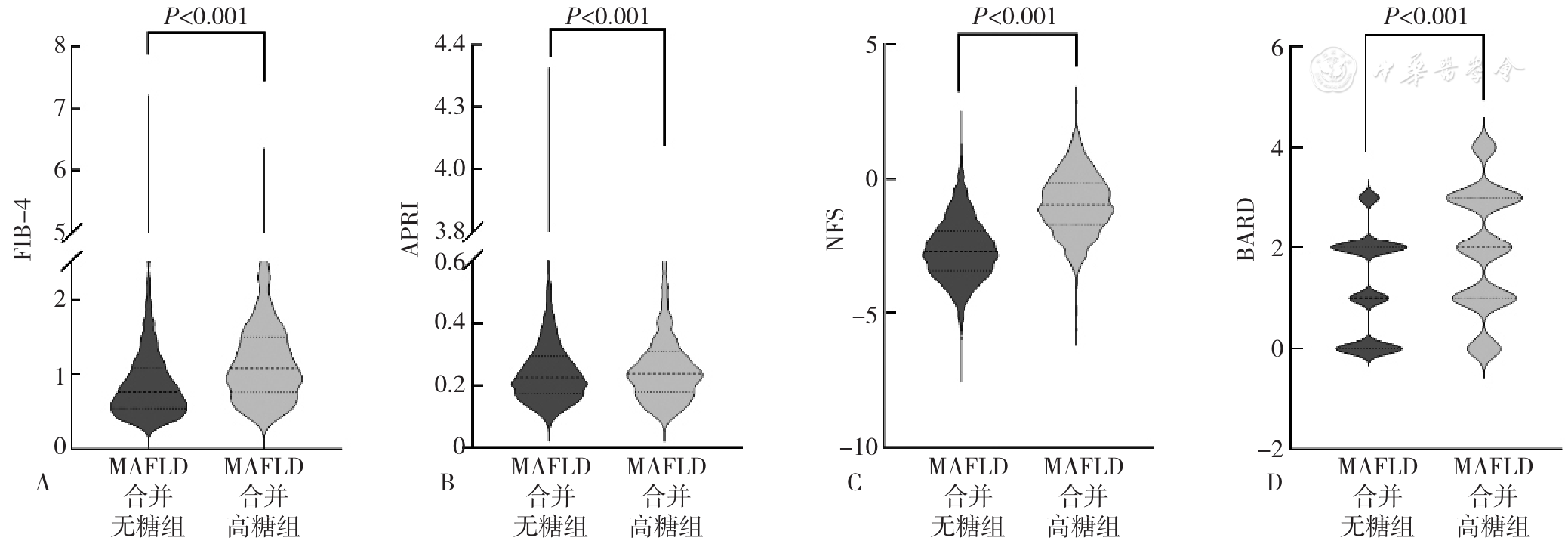
图2 MAFLD合并高糖组与MAFLD合并无糖组肝纤维化相关指数比较注:A为基于4因子的纤维化指数(FIB-4),B为天冬氨酸氨基转移酶/血小板计数比值(APRI),C为非酒精性脂肪肝肝纤维化评分(NFS),D为BMI、天冬氨酸氨基转移酶/丙氨酸氨基转移酶和糖尿病评分(BARD)。
Figure 2 Comparison of liver fibrosis related indices between MAFLD with hyperglycemia group and MAFLD without hyperglycemia group
| 组别 | 例数 | 性别(男/女) | 吸烟[例(%)] | 饮酒(无/偶尔/适量) | 高血压[例(%)] | 高脂血症[例(%)] | 高尿酸血症[例(%)] | 冠心病[例(%)] | 年龄[M(P25,P75),岁] | BMI[M(P25,P75),kg/m2] |
|---|---|---|---|---|---|---|---|---|---|---|
| MAFLD合并无糖组 | 4 506 | 3 285/1 221 | 1 019(22.6) | 3 651/763/92 | 927(20.6) | 2 534(56.2) | 1 636(36.3) | 11(0.2) | 44(36,55) | 26.3(24.6,28.4) |
| MAFLD合并高糖组 | 752 | 576/176 | 274(36.4) | 589/146/17 | 374(49.7) | 503(66.9) | 195(25.9) | 8(1.1) | 59(48,66) | 26.3(24.5,29.0) |
| Z(χ2)值 | 4.505a | 66.393a | 3.028a | 294.310a | 29.972a | 30.571a | 12.027a | -20.529 | -1.003 | |
| P值 | 0.034 | <0.001 | 0.220 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.316 | |
| 组别 | WC[M(P25,P75),cm] | PLT[M(P25,P75),×109/L] | WBC[M(P25,P75),×109/L] | RBC[M(P25,P75),×1012/L] | Hb[M(P25,P75),g/L] | RDW[M(P25,P75),%] | NEUT[M(P25,P75),×109/L] | LYM[M(P25,P75),×109/L] | MONO[M(P25,P75),×109/L] | |
| MAFLD合并无糖组 | 87(82,93) | 239(205,277) | 6.4(5.5,7.4) | 5.0(4.7,5.3) | 150(140,158) | 12.5(12.1,12.9) | 3.5(2.9,4.2) | 2.2(1.8,2.6) | 0.5(0.4,0.6) | |
| MAFLD合并高糖组 | 89(83,95) | 225(186,263) | 6.7(5.7,7.8) | 5.0(4.6,5.3) | 150(141,158) | 12.5(12.2,13.0) | 3.7(3.0,4.6) | 2.2(1.8,2.6) | 0.5(0.4,0.6) | |
| Z(χ2)值 | -6.214 | -6.973 | -4.861 | -0.009 | -0.446 | -3.395 | -5.916 | -0.830 | -4.865 | |
| P值 | <0.001 | <0.001 | <0.001 | 0.992 | 0.655 | <0.001 | <0.001 | 0.406 | <0.001 | |
| 组别 | NEUT%[M(P25,P75),%] | ALT[M(P25,P75),U/L] | AST[M(P25,P75),U/L] | TC[M(P25,P75),mmol/L] | TG[M(P25,P75),mmol/L] | HDL-C[M(P25,P75),mmol/L] | LDL-C[M(P25,P75),mmol/L] | ALB[M(P25,P75),g/L] | ||
| MAFLD合并无糖组 | 54.9(49.8,59.9) | 26.4(18.8,39.0) | 21.0(17.5,26.0) | 5.0(4.4,5.6) | 1.8(1.3,2.5) | 1.1(0.9,1.2) | 3.0(2.5,3.6) | 44.5(43.0,46.0) | ||
| MAFLD合并高糖组 | 56.4(51.0,61.7) | 24.3(18.0,36.6) | 20.9(16.8,26.6) | 4.9(4.2,5.6) | 2.0(1.4,3.0) | 1.0(0.9,1.2) | 2.8(2.1,3.4) | 44.2(42.5,45.6) | ||
| Z(χ2)值 | -4.910 | -2.496 | -1.300 | -3.266 | -5.264 | -4.523 | -6.893 | -4.640 | ||
| P值 | <0.001 | 0.013 | 0.194 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| 组别 | GLB[M(P25,P75),g/L] | GGT[M(P25,P75),U/L] | UA[M(P25,P75),μmol/L] | Cr[M(P25,P75),μmol/L] | BUN[M(P25,P75),mmol/L] | TBIL[M(P25,P75),μmol/L] | DBIL[M(P25,P75),μmol/L] | IBIL[M(P25,P75),μmol/L] | ALP[M(P25,P75),U/L] | |
| MAFLD合并无糖组 | 29.4 (27.2,31.5) | 29.0 (20.9,43.3) | 390 (336,448) | 71.0 (60.0,79.6) | 5.1 (4.5,6.0) | 13.2 (10.4,16.7) | 3.7 (2.8,4.8) | 9.4 (7.4,12.0) | 74.0 (63.3,87.4) | |
| MAFLD合并高糖组 | 29.6 (27.2,32.2) | 31.0 (22.1,45.3) | 359 (303,422) | 68.0 (57.5,78.5) | 5.6 (4.8,6.8) | 13.5 (10.6,17.0) | 3.8 (2.8,5.1) | 9.8 (7.6,12.3) | 78.0 (64.3,93.1) | |
| Z(χ2)值 | -2.456 | -2.855 | -9.007 | -3.069 | -10.779 | -1.746 | -1.448 | -1.633 | -4.176 | |
| P值 | 0.014 | 0.004 | <0.001 | 0.002 | <0.001 | 0.081 | 0.148 | 0.102 | <0.001 |
表2 MAFLD合并高糖组及MAFLD合并无糖组研究对象一般资料比较
Table 2 Comparison of general data of subjects among groups with MAFLD with hyperglycemia and MAFLD without hyperglycemia patients
| 组别 | 例数 | 性别(男/女) | 吸烟[例(%)] | 饮酒(无/偶尔/适量) | 高血压[例(%)] | 高脂血症[例(%)] | 高尿酸血症[例(%)] | 冠心病[例(%)] | 年龄[M(P25,P75),岁] | BMI[M(P25,P75),kg/m2] |
|---|---|---|---|---|---|---|---|---|---|---|
| MAFLD合并无糖组 | 4 506 | 3 285/1 221 | 1 019(22.6) | 3 651/763/92 | 927(20.6) | 2 534(56.2) | 1 636(36.3) | 11(0.2) | 44(36,55) | 26.3(24.6,28.4) |
| MAFLD合并高糖组 | 752 | 576/176 | 274(36.4) | 589/146/17 | 374(49.7) | 503(66.9) | 195(25.9) | 8(1.1) | 59(48,66) | 26.3(24.5,29.0) |
| Z(χ2)值 | 4.505a | 66.393a | 3.028a | 294.310a | 29.972a | 30.571a | 12.027a | -20.529 | -1.003 | |
| P值 | 0.034 | <0.001 | 0.220 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.316 | |
| 组别 | WC[M(P25,P75),cm] | PLT[M(P25,P75),×109/L] | WBC[M(P25,P75),×109/L] | RBC[M(P25,P75),×1012/L] | Hb[M(P25,P75),g/L] | RDW[M(P25,P75),%] | NEUT[M(P25,P75),×109/L] | LYM[M(P25,P75),×109/L] | MONO[M(P25,P75),×109/L] | |
| MAFLD合并无糖组 | 87(82,93) | 239(205,277) | 6.4(5.5,7.4) | 5.0(4.7,5.3) | 150(140,158) | 12.5(12.1,12.9) | 3.5(2.9,4.2) | 2.2(1.8,2.6) | 0.5(0.4,0.6) | |
| MAFLD合并高糖组 | 89(83,95) | 225(186,263) | 6.7(5.7,7.8) | 5.0(4.6,5.3) | 150(141,158) | 12.5(12.2,13.0) | 3.7(3.0,4.6) | 2.2(1.8,2.6) | 0.5(0.4,0.6) | |
| Z(χ2)值 | -6.214 | -6.973 | -4.861 | -0.009 | -0.446 | -3.395 | -5.916 | -0.830 | -4.865 | |
| P值 | <0.001 | <0.001 | <0.001 | 0.992 | 0.655 | <0.001 | <0.001 | 0.406 | <0.001 | |
| 组别 | NEUT%[M(P25,P75),%] | ALT[M(P25,P75),U/L] | AST[M(P25,P75),U/L] | TC[M(P25,P75),mmol/L] | TG[M(P25,P75),mmol/L] | HDL-C[M(P25,P75),mmol/L] | LDL-C[M(P25,P75),mmol/L] | ALB[M(P25,P75),g/L] | ||
| MAFLD合并无糖组 | 54.9(49.8,59.9) | 26.4(18.8,39.0) | 21.0(17.5,26.0) | 5.0(4.4,5.6) | 1.8(1.3,2.5) | 1.1(0.9,1.2) | 3.0(2.5,3.6) | 44.5(43.0,46.0) | ||
| MAFLD合并高糖组 | 56.4(51.0,61.7) | 24.3(18.0,36.6) | 20.9(16.8,26.6) | 4.9(4.2,5.6) | 2.0(1.4,3.0) | 1.0(0.9,1.2) | 2.8(2.1,3.4) | 44.2(42.5,45.6) | ||
| Z(χ2)值 | -4.910 | -2.496 | -1.300 | -3.266 | -5.264 | -4.523 | -6.893 | -4.640 | ||
| P值 | <0.001 | 0.013 | 0.194 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| 组别 | GLB[M(P25,P75),g/L] | GGT[M(P25,P75),U/L] | UA[M(P25,P75),μmol/L] | Cr[M(P25,P75),μmol/L] | BUN[M(P25,P75),mmol/L] | TBIL[M(P25,P75),μmol/L] | DBIL[M(P25,P75),μmol/L] | IBIL[M(P25,P75),μmol/L] | ALP[M(P25,P75),U/L] | |
| MAFLD合并无糖组 | 29.4 (27.2,31.5) | 29.0 (20.9,43.3) | 390 (336,448) | 71.0 (60.0,79.6) | 5.1 (4.5,6.0) | 13.2 (10.4,16.7) | 3.7 (2.8,4.8) | 9.4 (7.4,12.0) | 74.0 (63.3,87.4) | |
| MAFLD合并高糖组 | 29.6 (27.2,32.2) | 31.0 (22.1,45.3) | 359 (303,422) | 68.0 (57.5,78.5) | 5.6 (4.8,6.8) | 13.5 (10.6,17.0) | 3.8 (2.8,5.1) | 9.8 (7.6,12.3) | 78.0 (64.3,93.1) | |
| Z(χ2)值 | -2.456 | -2.855 | -9.007 | -3.069 | -10.779 | -1.746 | -1.448 | -1.633 | -4.176 | |
| P值 | 0.014 | 0.004 | <0.001 | 0.002 | <0.001 | 0.081 | 0.148 | 0.102 | <0.001 |
| 组别 | 例数 | 性别(男/女) | 吸烟[例(%)] | 饮酒(无/偶尔/适量) | 高血压[例(%)] | 高脂血症[例(%)] | 高尿酸血症[例(%)] | 冠心病[例(%)] | 年龄[M(P25,P75),岁] | BMI[M(P25,P75),kg/m2] |
|---|---|---|---|---|---|---|---|---|---|---|
| MAFLD合并高糖组 | 376 | 280/96 | 135(35.9) | 304/65/7 | 182(48.4) | 248(66.0) | 84(22.3) | 5(1.3) | 58(49,66) | 26.3(24.5,29.0) |
| MAFLD合并无糖组 | 2 253 | 1 654/599 | 524(23.3) | 1 850/364/39 | 456(20.2) | 1 273(56.5) | 808(35.9) | 8(0.4) | 45(36,56) | 26.2(24.6,28.4) |
| 组别 | WC[M(P25,P75),cm] | PLT[M(P25,P75),×109/L] | WBC[M(P25,P75),×109/L] | RBC[M(P25,P75),×1012/L] | Hb[M(P25,P75),g/L] | RDW[M(P25,P75),%] | NEUT[M(P25,P75),×109/L] | LYM[M(P25,P75),×109/L] | MONO[M(P25,P75),×109/L] | |
| MAFLD合并高糖组 | 89(83,95) | 223(187,263) | 6.6(5.7,7.8) | 5.0(4.6,5.3) | 150(141,159) | 12.5(12.2,13.0) | 3.7(3.1,4.6) | 2.2(1.8,2.6) | 0.5(0.4,0.6) | |
| MAFLD合并无糖组 | 87(82,93) | 239(204,276) | 6.3(5.5,7.3) | 5.0(4.7,5.3) | 150(140,158) | 12.5(12.1,12.9) | 3.4(2.8,4.2) | 2.2(1.8,2.6) | 0.5(0.4,0.6) | |
| 组别 | NEUT% [M(P25,P75),%] | ALT[M(P25,P75),U/L] | AST[M(P25,P75),U/L] | TC[M(P25,P75),mmol/L] | TG[M(P25,P75),mmol/L] | HDL-C [M(P25,P75),mmol/L] | LDL-C [M(P25,P75),mmol/L] | ALB[M(P25,P75),g/L] | GLB[M(P25,P75),g/L] | |
| MAFLD合并高糖组 | 56.8(51.3,61.7) | 25.0(18.0,38.0) | 21.0(17.0,27.4) | 4.8(4.1,5.5) | 2.0(1.4,3.0) | 1.0(0.9,1.2) | 2.8(2.1,3.4) | 44.2(42.3,45.6) | 29.8(27.2,32.2) | |
| MAFLD合并无糖组 | 54.8(49.7,59.8) | 26.5(18.8,38.3) | 21.0(17.5,26.0) | 5.0(4.4,5.6) | 1.8(1.3,2.5) | 1.1(0.9,1.2) | 3.0(2.5,3.6) | 44.5(43.0,46.0) | 29.4(27.3,31.6) | |
| 组别 | GGT[M(P25,P75),U/L] | UA[M(P25,P75),μmol/L] | Cr[M(P25,P75),μmol/L] | BUN[M(P25,P75),mmol/L] | TBIL[M(P25,P75),μmol/L] | DBIL[M(P25,P75),μmol/L] | IBIL[M(P25,P75),μmol/L] | ALP[M(P25,P75),U/L] | ||
| MAFLD合并高糖组 | 32.2(22.3,46.7) | 352(303,420) | 68.8(57.5,78.9) | 5.7(4.9,6.8) | 13.7(10.8,17.4) | 3.9(2.9,5.1) | 9.8(7.6,12.3) | 80.4(65.0,96.0) | ||
| MAFLD合并无糖组 | 29.0(20.7,44.0) | 389(335,450) | 71.0(59.2,79.9) | 5.2(4.4,6.0) | 13.2(10.5,16.6) | 3.7(2.8,4.9) | 9.4(7.5,11.9) | 74.8(63.1,88.0) |
表3 训练集中MAFLD合并高糖组和MAFLD合并无糖组一般资料
Table 3 General data of subjects among groups with MAFLD with hyperglycemia and MAFLD without hyperglycemia patients in training set
| 组别 | 例数 | 性别(男/女) | 吸烟[例(%)] | 饮酒(无/偶尔/适量) | 高血压[例(%)] | 高脂血症[例(%)] | 高尿酸血症[例(%)] | 冠心病[例(%)] | 年龄[M(P25,P75),岁] | BMI[M(P25,P75),kg/m2] |
|---|---|---|---|---|---|---|---|---|---|---|
| MAFLD合并高糖组 | 376 | 280/96 | 135(35.9) | 304/65/7 | 182(48.4) | 248(66.0) | 84(22.3) | 5(1.3) | 58(49,66) | 26.3(24.5,29.0) |
| MAFLD合并无糖组 | 2 253 | 1 654/599 | 524(23.3) | 1 850/364/39 | 456(20.2) | 1 273(56.5) | 808(35.9) | 8(0.4) | 45(36,56) | 26.2(24.6,28.4) |
| 组别 | WC[M(P25,P75),cm] | PLT[M(P25,P75),×109/L] | WBC[M(P25,P75),×109/L] | RBC[M(P25,P75),×1012/L] | Hb[M(P25,P75),g/L] | RDW[M(P25,P75),%] | NEUT[M(P25,P75),×109/L] | LYM[M(P25,P75),×109/L] | MONO[M(P25,P75),×109/L] | |
| MAFLD合并高糖组 | 89(83,95) | 223(187,263) | 6.6(5.7,7.8) | 5.0(4.6,5.3) | 150(141,159) | 12.5(12.2,13.0) | 3.7(3.1,4.6) | 2.2(1.8,2.6) | 0.5(0.4,0.6) | |
| MAFLD合并无糖组 | 87(82,93) | 239(204,276) | 6.3(5.5,7.3) | 5.0(4.7,5.3) | 150(140,158) | 12.5(12.1,12.9) | 3.4(2.8,4.2) | 2.2(1.8,2.6) | 0.5(0.4,0.6) | |
| 组别 | NEUT% [M(P25,P75),%] | ALT[M(P25,P75),U/L] | AST[M(P25,P75),U/L] | TC[M(P25,P75),mmol/L] | TG[M(P25,P75),mmol/L] | HDL-C [M(P25,P75),mmol/L] | LDL-C [M(P25,P75),mmol/L] | ALB[M(P25,P75),g/L] | GLB[M(P25,P75),g/L] | |
| MAFLD合并高糖组 | 56.8(51.3,61.7) | 25.0(18.0,38.0) | 21.0(17.0,27.4) | 4.8(4.1,5.5) | 2.0(1.4,3.0) | 1.0(0.9,1.2) | 2.8(2.1,3.4) | 44.2(42.3,45.6) | 29.8(27.2,32.2) | |
| MAFLD合并无糖组 | 54.8(49.7,59.8) | 26.5(18.8,38.3) | 21.0(17.5,26.0) | 5.0(4.4,5.6) | 1.8(1.3,2.5) | 1.1(0.9,1.2) | 3.0(2.5,3.6) | 44.5(43.0,46.0) | 29.4(27.3,31.6) | |
| 组别 | GGT[M(P25,P75),U/L] | UA[M(P25,P75),μmol/L] | Cr[M(P25,P75),μmol/L] | BUN[M(P25,P75),mmol/L] | TBIL[M(P25,P75),μmol/L] | DBIL[M(P25,P75),μmol/L] | IBIL[M(P25,P75),μmol/L] | ALP[M(P25,P75),U/L] | ||
| MAFLD合并高糖组 | 32.2(22.3,46.7) | 352(303,420) | 68.8(57.5,78.9) | 5.7(4.9,6.8) | 13.7(10.8,17.4) | 3.9(2.9,5.1) | 9.8(7.6,12.3) | 80.4(65.0,96.0) | ||
| MAFLD合并无糖组 | 29.0(20.7,44.0) | 389(335,450) | 71.0(59.2,79.9) | 5.2(4.4,6.0) | 13.2(10.5,16.6) | 3.7(2.8,4.9) | 9.4(7.5,11.9) | 74.8(63.1,88.0) |
| 临床指标 | 单因素分析 | 多因素分析 | ||
|---|---|---|---|---|
| OR(95%CI) | P值 | OR(95%CI) | P值 | |
| 性别 | 1.200(0.931~1.546) | 0.159 | ||
| 吸烟 | 1.829(1.447~2.312) | <0.001 | ||
| 饮酒 | 1.073(0.842~1.367) | 0.570 | ||
| 高血压 | 4.127(3.286~5.182) | <0.001 | 2.150(1.647~2.808) | <0.001 |
| 高脂血症 | 1.709(1.356~2.155) | <0.001 | 1.639(1.237~2.172) | <0.001 |
| 高尿酸血症 | 0.599(0.467~0.767) | <0.001 | ||
| 冠心病 | 6.059(1.746~21.032) | 0.005 | ||
| 年龄 | 1.063(1.054~1.073) | <0.001 | 1.047(1.036~1.058) | <0.001 |
| BMI | 1.022(0.988~1.057) | 0.205 | ||
| WC | 1.028(1.016~1.041) | <0.001 | 1.036(1.021~1.052) | <0.001 |
| PLT | 0.996(0.994~0.998) | <0.001 | ||
| WBC | 1.157(1.081~1.239) | <0.001 | ||
| RBC | 1.043(0.821~1.324) | 0.732 | ||
| Hb | 1.005(0.997~1.013) | 0.251 | ||
| RDW | 1.055(0.952~1.169) | 0.305 | ||
| NEUT | 1.258(1.151~1.375) | <0.001 | ||
| LYM | 0.985(0.825~1.176) | 0.864 | ||
| MONO | 3.911(1.800~8.497) | <0.001 | ||
| NEUT% | 1.030(1.016~1.045) | <0.001 | ||
| ALT | 0.997(0.993~1.002) | 0.300 | ||
| AST | 1.003(0.994~1.012) | 0.493 | ||
| TC | 0.767(0.680~0.866) | <0.001 | ||
| TG | 1.182(1.112~1.256) | <0.001 | 1.176(1.093~1.265) | <0.001 |
| HDL-C | 0.307(0.179~0.527) | <0.001 | ||
| LDL-C | 0.612(0.532~0.704) | <0.001 | ||
| ALB | 0.921(0.877~0.967) | <0.001 | ||
| GLB | 1.029(0.997~1.061) | 0.073 | ||
| GGT | 1.004(1.001~1.007) | 0.003 | 1.005(1.002~1.009) | <0.001 |
| UA | 0.995(0.994~0.997) | <0.001 | 0.993(0.992~0.995) | <0.001 |
| Cr | 0.996(0.988~1.003) | 0.221 | ||
| BUN | 1.504(1.382~1.637) | <0.001 | 1.321(1.201~1.454) | <0.001 |
| TBIL | 1.014(0.995~1.033) | 0.144 | ||
| DBIL | 1.056(0.994~1.122) | 0.077 | ||
| IBIL | 1.016(0.991~1.043) | 0.217 | ||
| ALP | 1.011(1.007~1.016) | <0.001 | ||
表4 MAFLD合并高血糖影响因素的Logistic回归分析
Table 4 Logistic regression analysis of factors influencing MAFLD with hyperglycemia
| 临床指标 | 单因素分析 | 多因素分析 | ||
|---|---|---|---|---|
| OR(95%CI) | P值 | OR(95%CI) | P值 | |
| 性别 | 1.200(0.931~1.546) | 0.159 | ||
| 吸烟 | 1.829(1.447~2.312) | <0.001 | ||
| 饮酒 | 1.073(0.842~1.367) | 0.570 | ||
| 高血压 | 4.127(3.286~5.182) | <0.001 | 2.150(1.647~2.808) | <0.001 |
| 高脂血症 | 1.709(1.356~2.155) | <0.001 | 1.639(1.237~2.172) | <0.001 |
| 高尿酸血症 | 0.599(0.467~0.767) | <0.001 | ||
| 冠心病 | 6.059(1.746~21.032) | 0.005 | ||
| 年龄 | 1.063(1.054~1.073) | <0.001 | 1.047(1.036~1.058) | <0.001 |
| BMI | 1.022(0.988~1.057) | 0.205 | ||
| WC | 1.028(1.016~1.041) | <0.001 | 1.036(1.021~1.052) | <0.001 |
| PLT | 0.996(0.994~0.998) | <0.001 | ||
| WBC | 1.157(1.081~1.239) | <0.001 | ||
| RBC | 1.043(0.821~1.324) | 0.732 | ||
| Hb | 1.005(0.997~1.013) | 0.251 | ||
| RDW | 1.055(0.952~1.169) | 0.305 | ||
| NEUT | 1.258(1.151~1.375) | <0.001 | ||
| LYM | 0.985(0.825~1.176) | 0.864 | ||
| MONO | 3.911(1.800~8.497) | <0.001 | ||
| NEUT% | 1.030(1.016~1.045) | <0.001 | ||
| ALT | 0.997(0.993~1.002) | 0.300 | ||
| AST | 1.003(0.994~1.012) | 0.493 | ||
| TC | 0.767(0.680~0.866) | <0.001 | ||
| TG | 1.182(1.112~1.256) | <0.001 | 1.176(1.093~1.265) | <0.001 |
| HDL-C | 0.307(0.179~0.527) | <0.001 | ||
| LDL-C | 0.612(0.532~0.704) | <0.001 | ||
| ALB | 0.921(0.877~0.967) | <0.001 | ||
| GLB | 1.029(0.997~1.061) | 0.073 | ||
| GGT | 1.004(1.001~1.007) | 0.003 | 1.005(1.002~1.009) | <0.001 |
| UA | 0.995(0.994~0.997) | <0.001 | 0.993(0.992~0.995) | <0.001 |
| Cr | 0.996(0.988~1.003) | 0.221 | ||
| BUN | 1.504(1.382~1.637) | <0.001 | 1.321(1.201~1.454) | <0.001 |
| TBIL | 1.014(0.995~1.033) | 0.144 | ||
| DBIL | 1.056(0.994~1.122) | 0.077 | ||
| IBIL | 1.016(0.991~1.043) | 0.217 | ||
| ALP | 1.011(1.007~1.016) | <0.001 | ||
| 联合模型 | 金标准 | 合计 | |
|---|---|---|---|
| 诊断为MAFLD合并高血糖 | 诊断为MAFLD合并无糖 | ||
| 预测为MAFLD合并高血糖 | 265 | 607 | 872 |
| 预测为MAFLD合并无糖 | 111 | 1 646 | 1 757 |
| 合计 | 376 | 2 253 | 2 629 |
表5 联合模型预测MAFLD合并高血糖发生的效能验证(例)
Table 5 Validation of the predictive efficacy of the combined model for the occurrence of MAFLD complicated with hyperglycemia
| 联合模型 | 金标准 | 合计 | |
|---|---|---|---|
| 诊断为MAFLD合并高血糖 | 诊断为MAFLD合并无糖 | ||
| 预测为MAFLD合并高血糖 | 265 | 607 | 872 |
| 预测为MAFLD合并无糖 | 111 | 1 646 | 1 757 |
| 合计 | 376 | 2 253 | 2 629 |
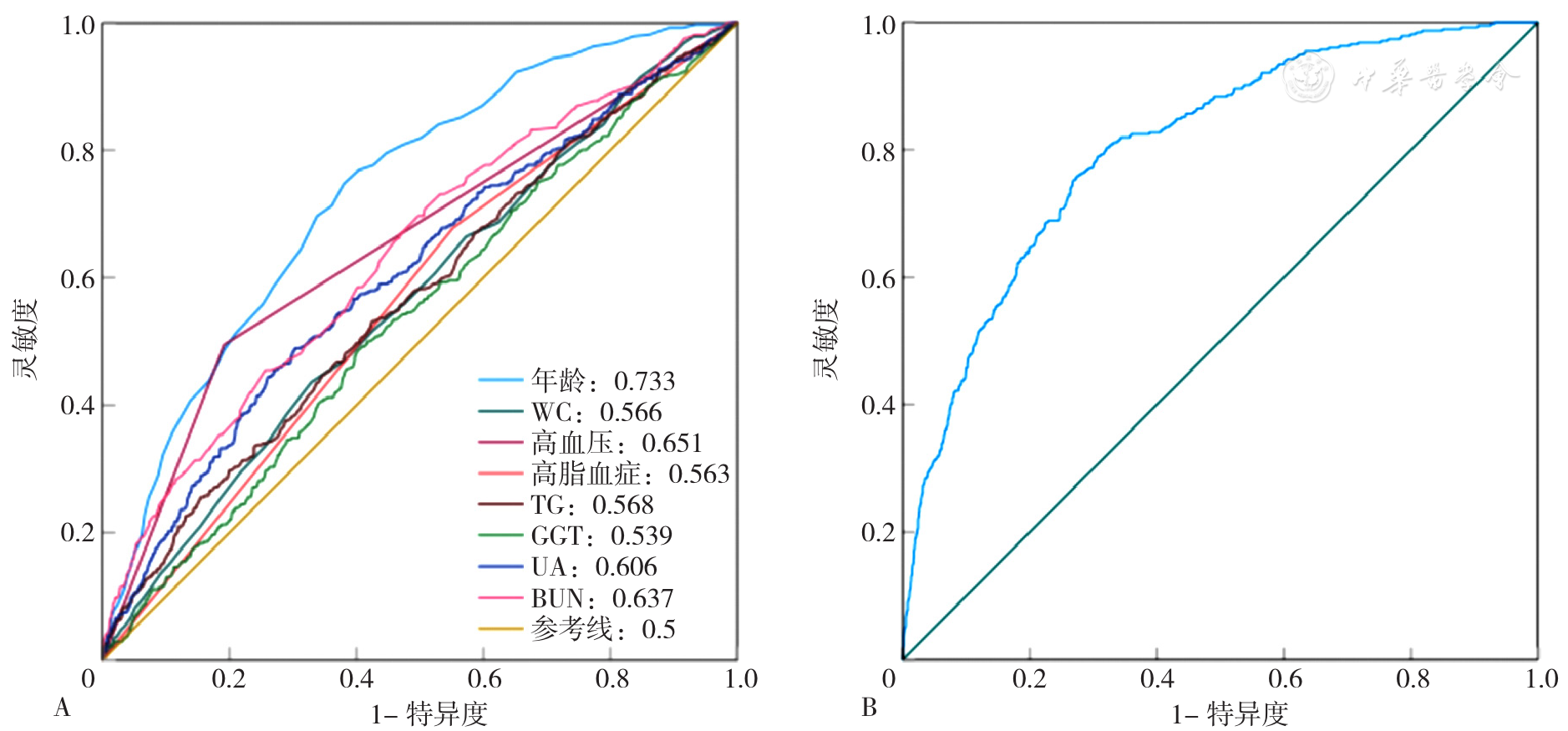
图3 各独立预测因子和联合模型预测MAFLD合并高血糖发生的ROC曲线注:A为各独立预测因子,B为联合模型;WC=腰围,TG=三酰甘油,GGT=γ-谷氨酰转肽酶,UA=尿酸,BUN=尿素氮。
Figure 3 The ROC curves of each independent predictor and the combined model for predicting the occurrence of MAFLD combined with hyperglycemia
| [1] |
中华医学会肝病学分会. 代谢相关(非酒精性)脂肪性肝病防治指南(2024年版)[J]. 中华肝脏病杂志,2024,32(5):418-434. DOI:10.3760/cma.j.cn501113-20240327-00163.
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] | |
| [7] |
施晓英,傅思华,傅怡悦. NAFLD患者肝纤维化影响因素的风险预测模型构建与效能评价[J]. 现代实用医学,2024,36(3):327-330.
|
| [8] |
郭滔,胡波,易为民,等. 血清学诊断非酒精性脂肪性肝病的研究进展[J]. 临床肝胆病杂志,2020,36(11):2579-2583. DOI:10.3969/j.issn.1001-5256.2020.11.041.
|
| [9] |
|
| [10] |
孔德先,邢煜玲,孙文文,等. 预估葡萄糖处理率与2型糖尿病合并代谢相关脂肪性肝病的相关性研究[J]. 中国全科医学,2023,26(26):3252-3258. DOI:10.12114/j.issn.1007-9572.2023.0103.
|
| [11] |
|
| [12] |
|
| [13] |
张迪,孙侃,刘宇,等. 脂肪肝指数在非酒精性脂肪性肝病诊断中的应用价值[J]. 内科理论与实践,2012,7(3):201-204. DOI:10.3969/j.issn.1673-6087.2012.03.013.
|
| [14] |
|
| [15] |
|
| [16] |
舒筠然,李俊琪,刘琼. 非酒精性脂肪性肝病的流行病学和危险因素分析[J]. 临床肝胆病杂志,2019,35(9):2085-2090. DOI:10.3969/j.issn.1001-5256.2019.09.045.
|
| [17] |
钱方方,戴梅清,赵丽,等. 血清碱性磷酸酶与2型糖尿病合并非酒精性脂肪肝病的相关性分析[J]. 临床肝胆病杂志,2023,39(1):83-88. DOI:10.3969/j.issn.1001-5256.2023.01.013.
|
| [18] |
|
| [19] |
|
| [20] |
|
| [1] | 周倩, 吴晓敏, 王宝华, 严若菡, 蔚苗, 吴静. 胃癌发生风险的列线图预测模型研究[J]. 中国全科医学, 2025, 28(23): 2870-2877. |
| [2] | 刘月影, 王雪丽, 刘雨秋, 魏立民. 空腹C肽与糖尿病病程比值与2型糖尿病发生代谢相关脂肪性肝病的相关性研究[J]. 中国全科医学, 2025, 28(23): 2852-2860. |
| [3] | 王鹏, 仇丽霞, 许姗姗, 张洋, 张晶, 杜晓菲. 代谢相关脂肪性肝病与2型糖尿病共同管理研究进展[J]. 中国全科医学, 2025, 28(23): 2846-2851. |
| [4] | 白雪, 陈倩倩, 李婕. 慢病管理创新实践:糖肝全-专管理新模式[J]. 中国全科医学, 2025, 28(23): 2841-2845. |
| [5] | 张冰清, 王忠凯, 吴长勇, 孙煌, 李锐洁, 刘文洁, 骆怡哗, 郑丽慧, 彭云珠. 1990—2021年全球先天性心脏缺陷疾病负担变化及未来趋势预测研究[J]. 中国全科医学, 2025, 28(18): 2253-2261. |
| [6] | 聂媛媛, 方达, 徐浩, 杨东辉, 毕艳, 顾天伟. 不同肝纤维化风险的2型糖尿病患者临床特征及心血管疾病风险研究[J]. 中国全科医学, 2025, 28(15): 1847-1854. |
| [7] | 卓莉莉, 瞿欢佳, 张秋玲. 2型糖尿病与肥胖对纤维化4指数筛查非酒精性脂肪性肝病早期肝纤维化影响的研究[J]. 中国全科医学, 2025, 28(11): 1354-1360. |
| [8] | 陈胜蓝, 郑永韬, 胡旺成, 倪作为, 夏冰, 叶春梅, 杜持新, 陈晓丹. 中小学生高度近视发生风险预测模型:基于巢式病例对照研究[J]. 中国全科医学, 2025, 28(09): 1115-1121. |
| [9] | 中华预防医学会糖尿病预防与控制专业委员会. 中国糖尿病行为与生活方式干预指南(2024版)[J]. 中国全科医学, 2025, 28(07): 777-796. |
| [10] | 石小天, 王珊, 杨华昱, 杨一帆, 李旭, 窦国泽, 马清. 基于血常规炎性指标构建衰弱/衰弱前期发生风险列线图模型研究[J]. 中国全科医学, 2025, 28(05): 587-593. |
| [11] | 杜慧杰, 刘星雨, 徐明欢, 杨学智, 张慧琴, 莫佳丽, 卢依, 况杰. 急性缺血性脑卒中预后预测研究的应用进展:以机器学习预测模型为例[J]. 中国全科医学, 2025, 28(05): 554-560. |
| [12] | 岳海涛, 何婵婵, 成羽攸, 张森诚, 吴悠, 马晶. 基于机器学习的冠心病风险预测模型构建与比较[J]. 中国全科医学, 2025, 28(04): 499-509. |
| [13] | 张高钰, 王子涵, 高雪菲, 张瑾, 代天顾, 何清, 樊佳溶, 黄力, 李琳. 基于三酰甘油葡萄糖指数联合血管弹性指标的绝经后女性高血压患者冠心病发生风险模型开发研究[J]. 中国全科医学, 2025, 28(01): 39-46. |
| [14] | 周镇森, 黄岩, 程思为, 张小玉, 张晓雨, 孙婷, 杨先军, 谢晖, 马祖长. 早期主动脉硬化风险筛查模型的构建及验证研究[J]. 中国全科医学, 2024, 27(33): 4147-4154. |
| [15] | 任凌萱, 卢子琪, 齐威, 冯志杰. 基于单细胞转录组学测序的巨噬细胞在肝硬化-肝癌疾病进展中的功能研究[J]. 中国全科医学, 2024, 27(29): 3654-3663. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||





