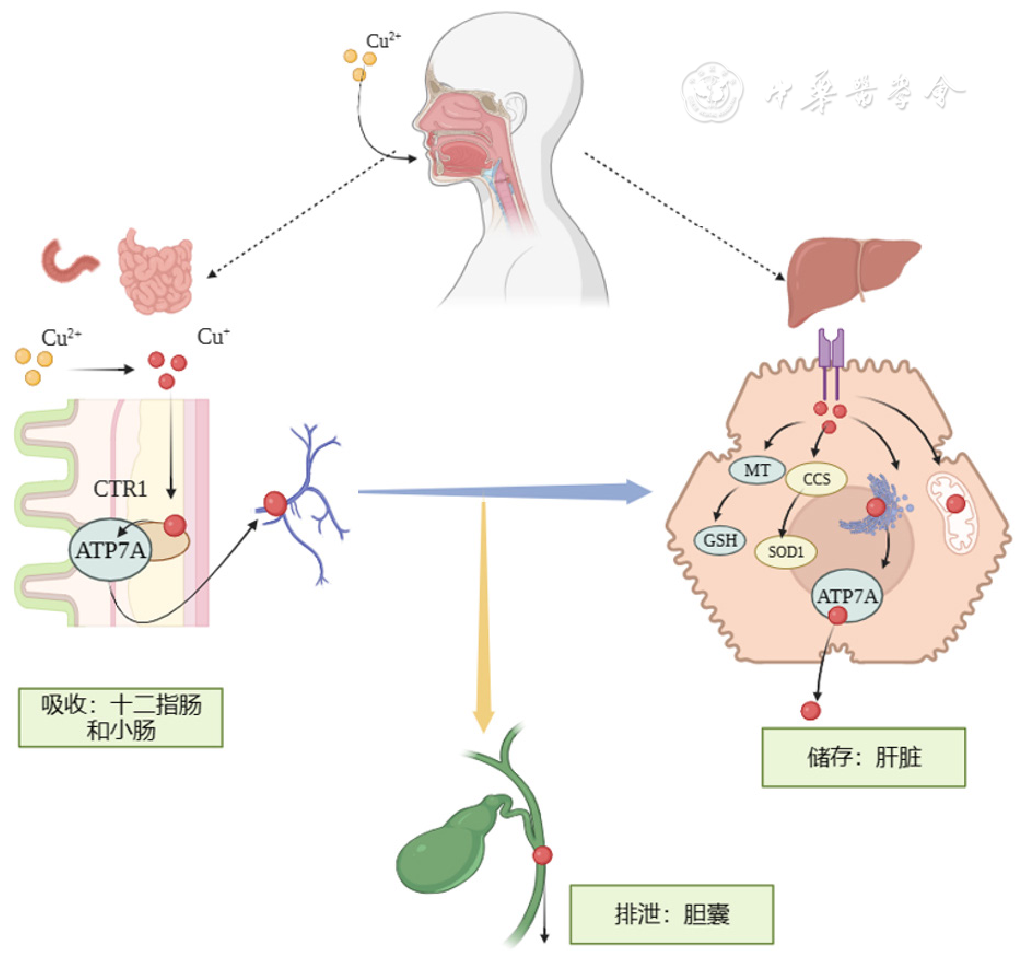
| [8] |
DAS A, ASH D, FOUDA A Y, et al. Cysteine oxidation of copper transporter CTR1 drives VEGFR2 signalling and angiogenesis[J]. Nat Cell Biol, 2022, 24(1):35-50. DOI: 10.1038/s41556-021-00822-7.
|
| [9] |
CHEN X Y, CAI Q, LIANG R K, et al. Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies[J]. Cell Death Dis, 2023, 14(2):105. DOI: 10.1038/s41419-023-05639-w.
|
| [10] |
TANG D L, CHEN X, KROEMER G. Cuproptosis:a copper-triggered modality of mitochondrial cell death[J]. Cell Res, 2022, 32(5):417-418. DOI: 10.1038/s41422-022-00653-7.
|
| [11] |
MANGALMURTI A, LUKENS J R. How neurons die in Alzheimer's disease:implications for neuroinflammation[J]. Curr Opin Neurobiol, 2022, 75:102575. DOI: 10.1016/j.conb.2022.102575.
|
| [12] |
FUKAI T, USHIO-FUKAI M, KAPLAN J H. Copper transporters and copper chaperones:roles in cardiovascular physiology and disease[J]. Am J Physiol Cell Physiol, 2018, 315(2):C186-201. DOI: 10.1152/ajpcell.00132.2018.
|
| [13] |
BOULLATA J, MUTHUKUMARAN G, PIARULLI A, et al. Oral copper absorption in men with morbid obesity[J]. J Trace Elem Med Biol, 2017, 44:146-150. DOI: 10.1016/j.jtemb.2017.07.005.
|
| [14] |
NISHITO Y, KAMBE T. Absorption mechanisms of iron,copper,and zinc:an overview[J]. J Nutr Sci Vitaminol, 2018, 64(1):1-7. DOI: 10.3177/jnsv.64.1.
|
| [15] |
MAGISTRATO A, PAVLIN M, QASEM Z, et al. Copper trafficking in eukaryotic systems:current knowledge from experimental and computational efforts[J]. Curr Opin Struct Biol, 2019, 58:26-33. DOI: 10.1016/j.sbi.2019.05.002.
|
| [16] |
KUO M T, CHEN H H W, SONG I S, et al. The roles of copper transporters in cisplatin resistance[J]. Cancer Metastasis Rev, 2007, 26(1):71-83. DOI: 10.1007/s10555-007-9045-3.
|
| [17] |
LIN C, ZHANG Z, WANG T, et al. Copper uptake by DMT1:a compensatory mechanism for CTR1 deficiency in human umbilical vein endothelial cells[J]. Metallomics, 2015, 7(8):1285-1289. DOI: 10.1039/c5mt00097a.
|
| [18] |
LIU Y, MIAO J. An emerging role of defective copper metabolism in heart disease[J]. Nutrients, 2022, 14(3):700. DOI: 10.3390/nu14030700.
|
| [19] |
POLISHCHUK E V, CONCILLI M, IACOBACCI S, et al. Wilson disease protein ATP7B utilizes lysosomal exocytosis to maintain copper homeostasis[J]. Dev Cell, 2014, 29(6):686-700. DOI: 10.1016/j.devcel.2014.04.033.
|
| [20] |
CHEN Z Y, LI Y Y, LIU X J. Copper homeostasis and copper-induced cell death:novel targeting for intervention in the pathogenesis of vascular aging[J]. Biomed Pharmacother, 2023, 169:115839. DOI: 10.1016/j.biopha.2023.115839.
|
| [21] |
TSVETKOV P, COY S, PETROVA B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins[J]. Science, 2022, 375(6586):1254-1261. DOI: 10.1126/science.abf0529.
|
| [22] |
NI M, SOLMONSON A, PAN C X, et al. Functional assessment of lipoyltransferase-1 deficiency in cells,mice,and humans[J]. Cell Rep, 2019, 27(5):1376-1386.e6. DOI: 10.1016/j.celrep.2019.04.005.
|
| [23] |
MAZHARI S, ARJMAND S, ESLAMI SHAHRBABAKI M, et al. Comparing copper serum level and cognitive functioning in patients with schizophrenia and healthy controls[J]. Basic Clin Neurosci, 2020, 11(5):649-657. DOI: 10.32598/bcn.9.10.11.5.2116.1.
|
| [24] |
KAZEMI T, MOODI M, RAJABI S, et al. Trace element concentration and cognitive dysfunction in elderly residents in Birjand[J]. Curr Alzheimer Res, 2022. DOI: 10.2174/1567205019666220913114154.
|
| [25] |
GONG Z L, SONG W L, GU M J. Serum copper and zinc concentrations and cognitive impairment in older adults aged 60 years and older[J]. Biol Trace Elem Res, 2022, 200(4):1495-1501. DOI: 10.1007/s12011-021-02765-4.
|
| [1] |
WONG M Y C, OU K L, CHUNG P K, et al. The relationship between physical activity,physical health,and mental health among older Chinese adults:a scoping review[J]. Front Public Health, 2023, 10:914548. DOI: 10.3389/fpubh.2022.914548.
|
| [2] |
TIWARI S, ATLURI V, KAUSHIK A, et al. Alzheimer's disease:pathogenesis,diagnostics,and therapeutics[J]. Int J Nanomedicine, 2019, 14:5541-5554. DOI: 10.2147/IJN.S200490.
|
| [3] |
LOI S M, PIJNENBURG Y, VELAKOULIS D. Recent research advances in young-onset dementia[J]. Curr Opin Psychiatry, 2023, 36(2):126-133. DOI: 10.1097/YCO.0000000000000843.
|
| [4] |
TANG W X, LI Y S, HE S X, et al. Caveolin-1 alleviates diabetes-associated cognitive dysfunction through modulating neuronal ferroptosis-mediated mitochondrial homeostasis[J]. Antioxid Redox Signal, 2022, 37(13/14/15):867-886. DOI: 10.1089/ars.2021.0233.
|
| [5] |
FU C, WU Y F, LIU S J, et al. Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia[J]. J Ethnopharmacol, 2022, 289:115021. DOI: 10.1016/j.jep.2022.115021.
|
| [6] |
ALAM A, HANA Z, JIN Z S, et al. Surgery,neuroinflammation and cognitive impairment[J]. EBioMedicine, 2018, 37:547-556. DOI: 10.1016/j.ebiom.2018.10.021.
|
| [7] |
TIAN Z M, JI X M, LIU J. Neuroinflammation in vascular cognitive impairment and dementia:current evidence,advances,and prospects[J]. Int J Mol Sci, 2022, 23(11):6224. DOI: 10.3390/ijms23116224.
|
| [26] |
DAVIES K M, MERCER J F, CHEN N, et al. Copper dyshomoeostasis in Parkinson's disease:implications for pathogenesis and indications for novel therapeutics[J]. Clin Sci, 2016, 130(8):565-574. DOI: 10.1042/CS20150153.
|
| [27] |
BUSH A I. Drug development based on the metals hypothesis of Alzheimer's disease[J]. J Alzheimers Dis, 2008, 15(2):223-240. DOI: 10.3233/jad-2008-15208.
|
| [28] |
CHERNY R A, AYTON S, FINKELSTEIN D I, et al. PBT2 reduces toxicity in a C. elegans model of polyQ aggregation and extends lifespan,reduces striatal atrophy and improves motor performance in the R6/2 mouse model of Huntington's disease[J]. J Huntingtons Dis, 2012, 1(2):211-219. DOI: 10.3233/JHD-120029.
|
| [29] |
CUI X N, WANG Y, LIU H, et al. The molecular mechanisms of defective copper metabolism in diabetic cardiomyopathy[J]. Oxid Med Cell Longev, 2022, 2022:5418376. DOI: 10.1155/2022/5418376.
|
| [30] |
LIN X M, WEI G, HUANG Z J, et al. Mitochondrial proteomic alterations caused by long-term low-dose copper exposure in mouse cortex[J]. Toxicol Lett, 2016, 263:16-25. DOI: 10.1016/j.toxlet.2016.10.009.
|
| [31] |
RUIZ L M, LIBEDINSKY A, ELORZA A A. Role of copper on mitochondrial function and metabolism[J]. Front Mol Biosci, 2021, 8:711227. DOI: 10.3389/fmolb.2021.711227.
|
| [32] |
SINGH I, SAGARE A P, COMA M, et al. Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance[J]. Proc Natl Acad Sci U S A, 2013, 110(36):14771-14776. DOI: 10.1073/pnas.1302212110.
|
| [33] |
SHRIBMAN S, POUJOIS A, BANDMANN O, et al. Wilson's disease:update on pathogenesis,biomarkers and treatments[J]. J Neurol Neurosurg Psychiatry, 2021, 92(10):1053-1061. DOI: 10.1136/jnnp-2021-326123.
|
| [34] |
TASSONE G, KOLA A, VALENSIN D, et al. Dynamic interplay between copper toxicity and mitochondrial dysfunction in Alzheimer's disease[J]. Life, 2021, 11(5):386. DOI: 10.3390/life11050386.
|
| [35] |
DUAN W J, HE R R. Cuproptosis:copper-induced regulated cell death[J]. Sci China Life Sci, 2022, 65(8):1680-1682. DOI: 10.1007/s11427-022-2106-6.
|
| [36] |
SHELINE C T, CHOI D W. Cu 2+ toxicity inhibition of mitochondrial dehydrogenases in vitro and in vivo[J]. Ann Neurol, 2004, 55(5):645-653. DOI: 10.1002/ana.20047.
|
| [37] |
ARCIELLO M, ROTILIO G, ROSSI L. Copper-dependent toxicity in SH-SY5Y neuroblastoma cells involves mitochondrial damage[J]. Biochem Biophys Res Commun, 2005, 327(2):454-459. DOI: 10.1016/j.bbrc.2004.12.022.
|
| [38] |
COBINE P A, BRADY D C. Cuproptosis:cellular and molecular mechanisms underlying copper-induced cell death[J]. Mol Cell, 2022, 82(10):1786-1787. DOI: 10.1016/j.molcel.2022.05.001.
|
| [39] |
CHEN L Y, MIN J X, WANG F D. Copper homeostasis and cuproptosis in health and disease[J]. Signal Transduct Target Ther, 2022, 7(1):378. DOI: 10.1038/s41392-022-01229-y.
|
| [40] |
陈桂琳,汤其强.阿尔茨海默病NK细胞与铜死亡的相关基因分析[J/OL].中国组织工程研究:1-9[2024-05-24].
|
| [41] |
MARTÍNEZ-HERNÁNDEZ M I, ACOSTA-SAAVEDRA L C, HERNÁNDEZ-KELLY L C, et al. Microglial activation in metal neurotoxicity:impact in neurodegenerative diseases[J]. Biomed Res Int, 2023, 2023:7389508. DOI: 10.1155/2023/7389508.
|
| [42] |
GUAN Y D, HAN F. Key mechanisms and potential targets of the NLRP3 inflammasome in neurodegenerative diseases[J]. Front Integr Neurosci, 2020, 14:37. DOI: 10.3389/fnint.2020.00037.
|
| [43] |
PAL A, KUMAR A, PRASAD R. Predictive association of copper metabolism proteins with Alzheimer's disease and Parkinson's disease:a preliminary perspective[J]. Biometals, 2014, 27(1):25-31. DOI: 10.1007/s10534-013-9702-7.
|
| [44] |
ZHOU Q, ZHANG Y, LU L, et al. Copper induces microglia-mediated neuroinflammation through ROS/NF-κB pathway and mitophagy disorder[J]. Food Chem Toxicol, 2022, 168:113369. DOI: 10.1016/j.fct.2022.113369.
|
| [45] |
CAETANO-SILVA M E, RUND L A, VAILATI-RIBONI M, et al. Copper-binding peptides attenuate microglia inflammation through suppression of NF-KB pathway[J]. Mol Nutr Food Res, 2021, 65(22):e2100153. DOI: 10.1002/mnfr.202100153.
|
| [46] |
LIAO Y, XING Q, LI Q Q, et al. Astrocytes in depression and Alzheimer's disease[J]. Front Med, 2021, 15(6):829-841. DOI: 10.1007/s11684-021-0875-0.
|
| [47] |
BHATTACHARJEE A, GHOSH S, CHATTERJI A, et al. Neuron-glia:understanding cellular copper homeostasis,its cross-talk and their contribution towards neurodegenerative diseases[J]. Metallomics, 2020, 12(12):1897-1911. DOI: 10.1039/d0mt00168f.
|
| [48] |
PIKE C J, CUMMINGS B J, MONZAVI R, et al. Beta-amyloid-induced changes in cultured astrocytes parallel reactive astrocytosis associated with senile plaques in Alzheimer's disease[J]. Neuroscience, 1994, 63(2):517-531. DOI: 10.1016/0306-4522(94)90547-9.
|
| [49] |
WITT B, STIBOLLER M, RASCHKE S, et al. Characterizing effects of excess copper levels in a human astrocytic cell line with focus on oxidative stress markers[J]. J Trace Elem Med Biol, 2021, 65:126711. DOI: 10.1016/j.jtemb.2021.126711.
|
| [50] |
COLOMBO E, TRIOLO D, BASSANI C, et al. Dysregulated copper transport in multiple sclerosis may cause demyelination via astrocytes[J]. Proc Natl Acad Sci USA, 2021, 118(27):e2025804118. DOI: 10.1073/pnas.2025804118.
|
| [51] |
GALE J R, HARTNETT-SCOTT K, ROSS M M, et al. Copper induces neuron-sparing,ferredoxin 1-independent astrocyte toxicity mediated by oxidative stress[J]. J Neurochem, 2023, 167(2):277-295. DOI: 10.1111/jnc.15961.
|
| [52] |
SCHEIBER I F, DRINGEN R. Copper-treatment increases the cellular GSH content and accelerates GSH export from cultured rat astrocytes[J]. Neurosci Lett, 2011, 498(1):42-46. DOI: 10.1016/j.neulet.2011.04.058.
|
| [53] |
OLIVERI V. Selective targeting of cancer cells by copper ionophores:an overview[J]. Front Mol Biosci, 2022, 9:841814. DOI: 10.3389/fmolb.2022.841814.
|
| [54] |
HUANG R, CHEN H, LIANG J Y, et al. Dual role of reactive oxygen species and their application in cancer therapy[J]. J Cancer, 2021, 12(18):5543-5561. DOI: 10.7150/jca.54699.
|
| [55] |
KALITA J, KUMAR V, MISRA U K. A study on apoptosis and anti-apoptotic status in Wilson disease[J]. Mol Neurobiol, 2016, 53(10):6659-6667. DOI: 10.1007/s12035-015-9570-y.
|
| [56] |
ANTONUCCI L, PORCU C, IANNUCCI G, et al. Non-alcoholic fatty liver disease and nutritional implications:special focus on copper[J]. Nutrients, 2017, 9(10):1137. DOI: 10.3390/nu9101137.
|
| [57] |
CUI L Y, GOUW A M, LAGORY E L, et al. Mitochondrial copper depletion suppresses triple-negative breast cancer in mice[J]. Nat Biotechnol, 2021, 39(3):357-367. DOI: 10.1038/s41587-020-0707-9.
|
| [58] |
|
| [59] |
CALABRESE V, GIORDANO J, SIGNORILE A, et al. Major pathogenic mechanisms in vascular dementia:roles of cellular stress response and hormesis in neuroprotection[J]. J Neurosci Res, 2016, 94(12):1588-1603. DOI: 10.1002/jnr.23925.
|
| [60] |
BHATIA P, SINGH N. Tadalafil ameliorates memory deficits,oxidative stress,endothelial dysfunction and neuropathological changes in rat model of hyperhomocysteinemia induced vascular dementia[J]. Int J Neurosci, 2022, 132(4):384-396. DOI: 10.1080/00207454.2020.1817009.
|
| [61] |
ZHU M L, ZHANG J, GUO L J, et al. Amorphous selenium inhibits oxidative stress injury of neurons in vascular dementia rats by activating NMDAR pathway[J]. Eur J Pharmacol, 2023, 955:175874. DOI: 10.1016/j.ejphar.2023.175874.
|
| [62] |
张颖. 铜暴露诱导认知障碍及神经毒性的机制研究[D]. 南京:东南大学,2022.
|
| [63] |
HUANG Y, XU W, ZHOU R B. NLRP3 inflammasome activation and cell death[J]. Cell Mol Immunol, 2021, 18(9):2114-2127. DOI: 10.1038/s41423-021-00740-6.
|
| [64] |
YU P, ZHANG X, LIU N, et al. Pyroptosis:mechanisms and diseases[J]. Signal Transduct Target Ther, 2021, 6(1):128. DOI: 10.1038/s41392-021-00507-5.
|
| [65] |
PAL A, RANI I, PAWAR A, et al. Microglia and astrocytes in Alzheimer's disease in the context of the aberrant copper homeostasis hypothesis[J]. Biomolecules, 2021, 11(11):1598. DOI: 10.3390/biom11111598.
|
| [66] |
AN Y N, ZHANG H F, WANG C, et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis[J]. FASEB J, 2019, 33(11):12515-12527. DOI: 10.1096/fj.201802805RR.
|
| [67] |
KELLEY N, JELTEMA D, DUAN Y H, et al. The NLRP3 inflammasome:an overview of mechanisms of activation and regulation[J]. Int J Mol Sci, 2019, 20(13):3328. DOI: 10.3390/ijms20133328.
|
| [68] |
SUN L, YONG Y, WEI P, et al. Electroacupuncture ameliorates postoperative cognitive dysfunction and associated neuroinflammation via NLRP3 signal inhibition in aged mice[J]. CNS Neurosci Ther, 2022, 28(3):390-400. DOI: 10.1111/cns.13784.
|
| [69] |
WANG H X, HE Y, SUN Z L, et al. Microglia in depression:an overview of microglia in the pathogenesis and treatment of depression[J]. J Neuroinflammation, 2022, 19(1):132. DOI: 10.1186/s12974-022-02492-0.
|
| [70] |
SELAKOVIC D, ALI D, EFTEKHARI A, et al. Editorial:Iatrogenic neurotoxicity - Mechanisms,prevention,and treatment[J]. Front Neurosci, 2023, 17:1184317. DOI: 10.3389/fnins.2023.1184317.
|
| [71] |
LIN X Y, CHEN Y R, ZHANG P, et al. The potential mechanism of postoperative cognitive dysfunction in older people[J]. Exp Gerontol, 2020, 130:110791. DOI: 10.1016/j.exger.2019.110791.
|
| [72] |
LIU J, LIU Y, WANG Y, et al. HMGB1 is a mediator of cuproptosis-related sterile inflammation[J]. Front Cell Dev Biol, 2022, 10:996307. DOI: 10.3389/fcell.2022.996307.
|
| [73] |
LISMAN J, COOPER K, SEHGAL M, et al. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability[J]. Nat Neurosci, 2018, 21(3):309-314. DOI: 10.1038/s41593-018-0076-6.
|
| [74] |
ZHANG Y, ZHOU Q, LU L, et al. Copper induces cognitive impairment in mice via modulation of cuproptosis and CREB signaling[J]. Nutrients, 2023, 15(4):972. DOI: 10.3390/nu15040972.
|
| [75] |
NALETOVA I, SATRIANO C, PIETROPAOLO A, et al. The copper(Ⅱ)-assisted connection between NGF and BDNF by means of nerve growth factor-mimicking short peptides[J]. Cells, 2019, 8(4):301. DOI: 10.3390/cells8040301.
|
| [76] |
MARINESCO S, CAREW T J. Serotonin release evoked by tail nerve stimulation in the CNS of aplysia:characterization and relationship to heterosynaptic plasticity[J]. J Neurosci, 2002, 22(6):2299-2312. DOI: 10.1523/JNEUROSCI.22-06-02299.2002.
|
| [77] |
BEN-ARI Y, GAIARSA J L, TYZIO R, et al. GABA:a pioneer transmitter that excites immature neurons and generates primitive oscillations[J]. Physiol Rev, 2007, 87(4):1215-1284. DOI: 10.1152/physrev.00017.2006.
|
| [78] |
KALITA J, KUMAR V, MISRA U K, et al. Memory and learning dysfunction following copper toxicity:biochemical and immunohistochemical basis[J]. Mol Neurobiol, 2018, 55(5):3800-3811. DOI: 10.1007/s12035-017-0619-y.
|
| [79] |
LI Z N, WANG G D, ZHONG S M, et al. Alleviation of cognitive deficits and high copper levels by an NMDA receptor antagonist in a rat depression model[J]. Compr Psychiatry, 2020, 102:152200. DOI: 10.1016/j.comppsych.2020.152200.
|
| [80] |
LI Y Q. Copper homeostasis:emerging target for cancer treatment[J]. IUBMB Life, 2020, 72(9):1900-1908. DOI: 10.1002/iub.2341.
|
| [81] |
AN Y M, LI S N, HUANG X Q, et al. The role of copper homeostasis in brain disease[J]. Int J Mol Sci, 2022, 23(22):13850. DOI: 10.3390/ijms232213850.
|
| [82] |
LIU T, LIU Y L, ZHANG F Y, et al. Copper homeostasis dysregulation promoting cell damage and the association with liver diseases[J]. Chin Med J, 2023, 136(14):1653-1662. DOI: 10.1097/CM9.0000000000002697.
|
 ), 董新刚1,3, 王晓元1
), 董新刚1,3, 王晓元1
 ), DONG Xingang1,3, WANG Xiaoyuan1
), DONG Xingang1,3, WANG Xiaoyuan1