

Chinese General Practice ›› 2025, Vol. 28 ›› Issue (14): 1795-1808.DOI: 10.12114/j.issn.1007-9572.2024.0384
• Original Research·Evidence-based Medicine·Traditional Chinese Medicine • Previous Articles
Received:2024-09-10
Revised:2024-12-13
Published:2025-05-15
Online:2025-03-06
Contact:
SUN Wenjun
通讯作者:
孙文军
作者简介:作者贡献:
朱胜杰负责文章的构思及设计,可行性分析,论文撰写;朱胜杰、杭晓屹进行数据收集,数据整理;朱胜杰、刁华琼进行统计学的处理,图、表的绘制与展示,结果的分析与解释;孙文军负责文章整体工作,全文的质量审校及监督管理。
基金资助:CLC Number:
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.chinagp.net/EN/10.12114/j.issn.1007-9572.2024.0384
| 第一作者 | 发表时间(年) | 样本量(例) | 年龄(岁) | 干预措施(T) | 疗程(d) | 结局指标 | ||
|---|---|---|---|---|---|---|---|---|
| T | C | T | C | |||||
| 常留军[ | 2020 | 41 | 41 | 59.93±5.13 | 58.03±5.20 | DSCXQ+常规西药 | 14 | ⑥⑦⑧ |
| 姚灵枝[ | 2018 | 45 | 45 | 63.0±6.0 | 62.0±6.5 | DSCXQ+常规西药 | 14 | ③④⑤⑥⑦ |
| 王飒[ | 2022 | 50 | 50 | 62.83±5.79 | 62.55±5.67 | DSCXQ+常规西药 | 14 | ③④⑤⑥⑦ |
| 张祖康[ | 2022 | 46 | 46 | 59.84±6.10 | 59.28±6.07 | DH+常规西药 | 14 | ③④⑤⑧ |
| 冯子凌[ | 2013 | 32 | 30 | 62.2±6.8 | 61.2±5.8 | DH+常规西药 | 15 | ③④⑤⑥⑦ |
| 徐波[ | 2017 | 48 | 48 | 52.36±9.81 | 53.83±7.98 | DH+常规西药 | 14 | ①⑥⑦⑧ |
| 王春玲[ | 2010 | 43 | 41 | 76±5.12 | 75±4.23 | DH+常规西药 | 10 | ⑥⑦ |
| 张勇[ | 2013 | 32 | 32 | 70.1±8.5 | 70.5±7.4 | SXPTT+常规西药 | 14 | ⑥⑦ |
| 邱红[ | 2013 | 40 | 42 | 42~75 | 40~76 | SXPTT+常规西药 | 14 | ③④⑤⑧ |
| 马良[ | 2017 | 60 | 60 | 55.8±8.6 | 55.5±8.4 | SM+常规西药 | 14 | ② |
| 肖展翅[ | 2014 | 38 | 37 | 54.68±8.37 | SM+常规西药 | 14 | ③④⑤⑥⑦⑧ | |
| 邢海辉[ | 2017 | 35 | 35 | 62.9±7.6 | 62.1±7.2 | SM+常规西药 | 14 | ③④⑤ |
| 刘俊花[ | 2024 | 41 | 41 | 49.52±8.27 | 49.27±8.31 | TMS+常规西药 | 14 | ②⑥⑧ |
| 宋晓丽[ | 2020 | 42 | 42 | 64.5±9.3 | 64.8±9.5 | TMS+常规西药 | 14 | ②③④⑤⑥⑦ |
| 周景来[ | 2010 | 60 | 60 | 63.8±8.4 | 64.7±8.1 | TMS+常规西药 | 14 | ③④⑤⑥⑦ |
| 相铁辉[ | 2020 | 59 | 59 | 62.01±3.91 | 62.13±4.04 | TMS+常规西药 | 14 | ②③④⑤⑧ |
| 韦正新[ | 2016 | 55 | 55 | 54.2±8.6 | 54.2±9.6 | TMS+常规西药 | 14 | ③④⑤⑧ |
| 吴迪[ | 2020 | 34 | 34 | 58.67±5.53 | 58.71±5.46 | TMS+常规西药 | 7 | ②③④⑤ |
| 方军涛[ | 2021 | 48 | 48 | 59.1±3.2 | 59.2±3.3 | TMS+常规西药 | 14 | ⑥⑦ |
| 叶斌[ | 2023 | 43 | 43 | 60.38±4.12 | 60.50±4.27 | TMS+常规西药 | 14 | ①⑥⑦ |
| 陈洁[ | 2023 | 36 | 36 | 48~80 | 45~76 | TMS+常规西药 | 未记录 | ⑥⑦ |
| 施建锋[ | 2017 | 40 | 40 | 64±8 | 65±6 | TMS+常规西药 | 14 | ③④⑤⑧ |
| 张岚[ | 2019 | 41 | 41 | 62.25±4.10 | 62.09±4.23 | TMS+常规西药 | 14 | ②⑧ |
| 唐铁钰[ | 2017 | 46 | 46 | 51.12±4.45 | 53.02±4.79 | TMS+常规西药 | 14 | ②③④⑤⑥⑦ |
| 陶海军[ | 2023 | 41 | 41 | 49.53±6.3 | 48.43±5.97 | TMS+常规西药 | 14 | ②③④⑤ |
| 曾利[ | 2012 | 58 | 50 | 53±5.3 | 55±4.3 | TMS+常规西药 | 14 | ③④⑤⑧ |
| 张春驰[ | 2011 | 42 | 41 | 65~83 | DZHS+常规西药 | 14 | ③④⑤⑥⑦ | |
| 郑泽荣[ | 2006 | 58 | 44 | 41~78 | 40~75 | DZHS+常规西药 | 10 | ③④⑤⑧ |
| 王美华[ | 2017 | 56 | 56 | 53.5±3.7 | 52.4±3.5 | SXT+常规西药 | 10 | ③④⑤ |
| 侯胜利[ | 2018 | 90 | 85 | 55.5±9.8 | 56.4±10.2 | SXT+常规西药 | 14 | ①③④⑤⑥⑦ |
| 郑素平[ | 2019 | 30 | 30 | 57.0±5.6 | 56.4±5.5 | HHHSS+常规西药 | 14 | ③④⑤⑥⑦⑧ |
| 李超[ | 2022 | 34 | 34 | 62.03±7.18 | 60.71±8.52 | HHHSS+常规西药 | 14 | ③④⑤⑥⑦⑧ |
| 赵耀[ | 2011 | 40 | 40 | 52.6±6.8 | SXN+常规西药 | 28 | ③④⑤ | |
| 周彩琴[ | 2018 | 50 | 50 | 58.32±0.68 | 57.36±0.64 | SXN+常规西药 | 1~14 | ③④⑤ |
| 谭永峰[ | 2022 | 40 | 40 | 64.79±6.27 | 64.53±5.81 | SXN+常规西药 | 14 | ②⑤ |
| 杨牧[ | 2019 | 75 | 75 | 60.5±11.5 | 62.5±11.5 | SXN+常规西药 | 14 | ③④⑤ |
| 任钦[ | 2015 | 60 | 60 | 77.4±9.5 | 77.4±9.6 | KDZ+常规西药 | 14 | ③④⑤ |
| 孙金柱[ | 2019 | 44 | 45 | 61.32±6.41 | 62.97±6.79 | KDZ+常规西药 | 未明确 | ③④⑤⑧ |
| 李嘉辉[ | 2028 | 47 | 47 | 59.82±6.75 | 60.84±3.18 | KDZ+常规西药 | 14 | ③④⑤⑧ |
| 王红洲[ | 2011 | 60 | 60 | 57.3±8.2 | 56.8±7.6 | GGS+常规西药 | 15 | ③④⑤⑥⑦⑧ |
| 简军[ | 2005 | 40 | 30 | 52.81±9.63 | 57.93±10.06 | GGS+常规西药 | 14 | ③④⑤⑥⑦⑧ |
| 杨明华[ | 2017 | 43 | 43 | 57.0±2.5 | 58.5±2.7 | GGS+常规西药 | 14 | ①③④⑤⑧ |
| 张方[ | 2020 | 51 | 51 | 60.89±6.10 | 58.10±7.38 | GGS+常规西药 | 14 | ③④⑤⑧ |
| 赵娜[ | 2019 | 60 | 60 | 72.5±4.9 | 71.2±4.5 | XNJ+常规西药 | 14 | ③④⑤ |
| 田丽丽[ | 2023 | 60 | 60 | 46.73±1.91 | 46.71±2.57 | XNJ+常规西药 | 14 | ② |
| 岳婷[ | 2014 | 43 | 41 | 59~85 | 63~84 | XNJ+常规西药 | 14 | ③④⑤⑥ |
| 温泽云[ | 2021 | 50 | 50 | 52.11±9.89 | 51.02±9.97 | YXDM+常规西药 | 48 | ②⑥⑦⑧ |
| 李法强[ | 2015 | 37 | 37 | 64.39±10.14 | 65.74±8.99 | YXDM+常规西药 | 10~14 | ①⑥⑧ |
| 栾琴[ | 2016 | 35 | 35 | 61.34±7.35 | 62.51±7.68 | YXDM+常规西药 | 14 | ①③④⑧ |
Table 1 Basic characteristics of included articles
| 第一作者 | 发表时间(年) | 样本量(例) | 年龄(岁) | 干预措施(T) | 疗程(d) | 结局指标 | ||
|---|---|---|---|---|---|---|---|---|
| T | C | T | C | |||||
| 常留军[ | 2020 | 41 | 41 | 59.93±5.13 | 58.03±5.20 | DSCXQ+常规西药 | 14 | ⑥⑦⑧ |
| 姚灵枝[ | 2018 | 45 | 45 | 63.0±6.0 | 62.0±6.5 | DSCXQ+常规西药 | 14 | ③④⑤⑥⑦ |
| 王飒[ | 2022 | 50 | 50 | 62.83±5.79 | 62.55±5.67 | DSCXQ+常规西药 | 14 | ③④⑤⑥⑦ |
| 张祖康[ | 2022 | 46 | 46 | 59.84±6.10 | 59.28±6.07 | DH+常规西药 | 14 | ③④⑤⑧ |
| 冯子凌[ | 2013 | 32 | 30 | 62.2±6.8 | 61.2±5.8 | DH+常规西药 | 15 | ③④⑤⑥⑦ |
| 徐波[ | 2017 | 48 | 48 | 52.36±9.81 | 53.83±7.98 | DH+常规西药 | 14 | ①⑥⑦⑧ |
| 王春玲[ | 2010 | 43 | 41 | 76±5.12 | 75±4.23 | DH+常规西药 | 10 | ⑥⑦ |
| 张勇[ | 2013 | 32 | 32 | 70.1±8.5 | 70.5±7.4 | SXPTT+常规西药 | 14 | ⑥⑦ |
| 邱红[ | 2013 | 40 | 42 | 42~75 | 40~76 | SXPTT+常规西药 | 14 | ③④⑤⑧ |
| 马良[ | 2017 | 60 | 60 | 55.8±8.6 | 55.5±8.4 | SM+常规西药 | 14 | ② |
| 肖展翅[ | 2014 | 38 | 37 | 54.68±8.37 | SM+常规西药 | 14 | ③④⑤⑥⑦⑧ | |
| 邢海辉[ | 2017 | 35 | 35 | 62.9±7.6 | 62.1±7.2 | SM+常规西药 | 14 | ③④⑤ |
| 刘俊花[ | 2024 | 41 | 41 | 49.52±8.27 | 49.27±8.31 | TMS+常规西药 | 14 | ②⑥⑧ |
| 宋晓丽[ | 2020 | 42 | 42 | 64.5±9.3 | 64.8±9.5 | TMS+常规西药 | 14 | ②③④⑤⑥⑦ |
| 周景来[ | 2010 | 60 | 60 | 63.8±8.4 | 64.7±8.1 | TMS+常规西药 | 14 | ③④⑤⑥⑦ |
| 相铁辉[ | 2020 | 59 | 59 | 62.01±3.91 | 62.13±4.04 | TMS+常规西药 | 14 | ②③④⑤⑧ |
| 韦正新[ | 2016 | 55 | 55 | 54.2±8.6 | 54.2±9.6 | TMS+常规西药 | 14 | ③④⑤⑧ |
| 吴迪[ | 2020 | 34 | 34 | 58.67±5.53 | 58.71±5.46 | TMS+常规西药 | 7 | ②③④⑤ |
| 方军涛[ | 2021 | 48 | 48 | 59.1±3.2 | 59.2±3.3 | TMS+常规西药 | 14 | ⑥⑦ |
| 叶斌[ | 2023 | 43 | 43 | 60.38±4.12 | 60.50±4.27 | TMS+常规西药 | 14 | ①⑥⑦ |
| 陈洁[ | 2023 | 36 | 36 | 48~80 | 45~76 | TMS+常规西药 | 未记录 | ⑥⑦ |
| 施建锋[ | 2017 | 40 | 40 | 64±8 | 65±6 | TMS+常规西药 | 14 | ③④⑤⑧ |
| 张岚[ | 2019 | 41 | 41 | 62.25±4.10 | 62.09±4.23 | TMS+常规西药 | 14 | ②⑧ |
| 唐铁钰[ | 2017 | 46 | 46 | 51.12±4.45 | 53.02±4.79 | TMS+常规西药 | 14 | ②③④⑤⑥⑦ |
| 陶海军[ | 2023 | 41 | 41 | 49.53±6.3 | 48.43±5.97 | TMS+常规西药 | 14 | ②③④⑤ |
| 曾利[ | 2012 | 58 | 50 | 53±5.3 | 55±4.3 | TMS+常规西药 | 14 | ③④⑤⑧ |
| 张春驰[ | 2011 | 42 | 41 | 65~83 | DZHS+常规西药 | 14 | ③④⑤⑥⑦ | |
| 郑泽荣[ | 2006 | 58 | 44 | 41~78 | 40~75 | DZHS+常规西药 | 10 | ③④⑤⑧ |
| 王美华[ | 2017 | 56 | 56 | 53.5±3.7 | 52.4±3.5 | SXT+常规西药 | 10 | ③④⑤ |
| 侯胜利[ | 2018 | 90 | 85 | 55.5±9.8 | 56.4±10.2 | SXT+常规西药 | 14 | ①③④⑤⑥⑦ |
| 郑素平[ | 2019 | 30 | 30 | 57.0±5.6 | 56.4±5.5 | HHHSS+常规西药 | 14 | ③④⑤⑥⑦⑧ |
| 李超[ | 2022 | 34 | 34 | 62.03±7.18 | 60.71±8.52 | HHHSS+常规西药 | 14 | ③④⑤⑥⑦⑧ |
| 赵耀[ | 2011 | 40 | 40 | 52.6±6.8 | SXN+常规西药 | 28 | ③④⑤ | |
| 周彩琴[ | 2018 | 50 | 50 | 58.32±0.68 | 57.36±0.64 | SXN+常规西药 | 1~14 | ③④⑤ |
| 谭永峰[ | 2022 | 40 | 40 | 64.79±6.27 | 64.53±5.81 | SXN+常规西药 | 14 | ②⑤ |
| 杨牧[ | 2019 | 75 | 75 | 60.5±11.5 | 62.5±11.5 | SXN+常规西药 | 14 | ③④⑤ |
| 任钦[ | 2015 | 60 | 60 | 77.4±9.5 | 77.4±9.6 | KDZ+常规西药 | 14 | ③④⑤ |
| 孙金柱[ | 2019 | 44 | 45 | 61.32±6.41 | 62.97±6.79 | KDZ+常规西药 | 未明确 | ③④⑤⑧ |
| 李嘉辉[ | 2028 | 47 | 47 | 59.82±6.75 | 60.84±3.18 | KDZ+常规西药 | 14 | ③④⑤⑧ |
| 王红洲[ | 2011 | 60 | 60 | 57.3±8.2 | 56.8±7.6 | GGS+常规西药 | 15 | ③④⑤⑥⑦⑧ |
| 简军[ | 2005 | 40 | 30 | 52.81±9.63 | 57.93±10.06 | GGS+常规西药 | 14 | ③④⑤⑥⑦⑧ |
| 杨明华[ | 2017 | 43 | 43 | 57.0±2.5 | 58.5±2.7 | GGS+常规西药 | 14 | ①③④⑤⑧ |
| 张方[ | 2020 | 51 | 51 | 60.89±6.10 | 58.10±7.38 | GGS+常规西药 | 14 | ③④⑤⑧ |
| 赵娜[ | 2019 | 60 | 60 | 72.5±4.9 | 71.2±4.5 | XNJ+常规西药 | 14 | ③④⑤ |
| 田丽丽[ | 2023 | 60 | 60 | 46.73±1.91 | 46.71±2.57 | XNJ+常规西药 | 14 | ② |
| 岳婷[ | 2014 | 43 | 41 | 59~85 | 63~84 | XNJ+常规西药 | 14 | ③④⑤⑥ |
| 温泽云[ | 2021 | 50 | 50 | 52.11±9.89 | 51.02±9.97 | YXDM+常规西药 | 48 | ②⑥⑦⑧ |
| 李法强[ | 2015 | 37 | 37 | 64.39±10.14 | 65.74±8.99 | YXDM+常规西药 | 10~14 | ①⑥⑧ |
| 栾琴[ | 2016 | 35 | 35 | 61.34±7.35 | 62.51±7.68 | YXDM+常规西药 | 14 | ①③④⑧ |
| 干预措施 | SM+常规西药 | TMS+常规西药 | SXN+常规西药 | XNJ+常规西药 | YXDM+常规西药 |
|---|---|---|---|---|---|
| TMS+常规西药 | -2.66(-11.58~6.25) | ||||
| SXN+常规西药 | -2.41(-14.00~9.17) | 0.25(-8.34~8.83) | |||
| XNJ+常规西药 | 2.67(-9.03~14.36) | 5.33(-3.40~14.05) | 5.08(-6.36~16.52) | ||
| YXDM+常规西药 | -11.01(-22.53~0.51) | -8.35(-16.84~0.14) | -8.60(-19.87~2.67) | -13.68(-25.05~-2.30)a | |
| 常规西药 | -14.64(-23.01~-6.28)a | -11.98(-15.06~-8.91)a | -12.23(-20.25~-4.21)a | -17.31(-25.48~-9.14)a | -3.63(-11.55~4.29) |
Table 2 Network meta-analysis of DHI scores in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | SM+常规西药 | TMS+常规西药 | SXN+常规西药 | XNJ+常规西药 | YXDM+常规西药 |
|---|---|---|---|---|---|
| TMS+常规西药 | -2.66(-11.58~6.25) | ||||
| SXN+常规西药 | -2.41(-14.00~9.17) | 0.25(-8.34~8.83) | |||
| XNJ+常规西药 | 2.67(-9.03~14.36) | 5.33(-3.40~14.05) | 5.08(-6.36~16.52) | ||
| YXDM+常规西药 | -11.01(-22.53~0.51) | -8.35(-16.84~0.14) | -8.60(-19.87~2.67) | -13.68(-25.05~-2.30)a | |
| 常规西药 | -14.64(-23.01~-6.28)a | -11.98(-15.06~-8.91)a | -12.23(-20.25~-4.21)a | -17.31(-25.48~-9.14)a | -3.63(-11.55~4.29) |
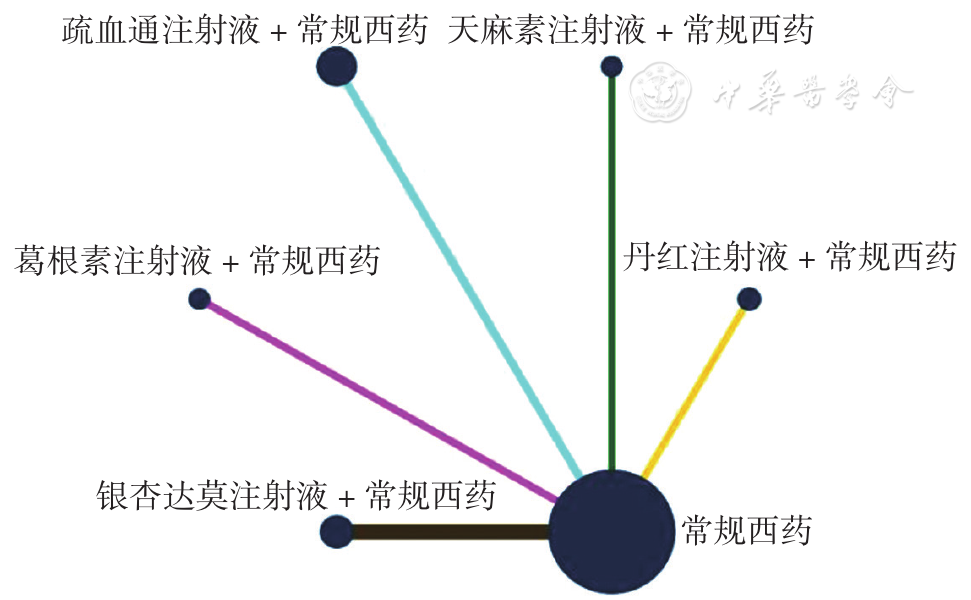
Figure 4 Network relationship diagram of the total clinical effective rate in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | DH+常规西药 | TMS+常规西药 | SXT+常规西药 | GGS+常规西药 | YXDM+常规西药 |
|---|---|---|---|---|---|
| TMS+常规西药 | 0.54(0.08~3.54) | ||||
| SXT+常规西药 | 0.77(0.17~3.39) | 1.41(0.29~6.77) | |||
| GGS+常规西药 | 0.62(0.09~4.09) | 1.14(0.16~8.03) | 0.81(0.17~3.94) | ||
| YXDM+常规西药 | 0.27(0.04~1.66) | 0.50(0.08~3.26) | 0.35(0.08~1.57) | 0.44(0.07~2.87) | |
| 常规西药 | 2.20(0.62~7.87) | 4.04(1.03~15.90)a | 2.86(1.34~6.14)a | 3.53(0.88~14.09) | 8.09(2.25~29.12)a |
Table 3 Network meta-analysis of the total clinical effective rate in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | DH+常规西药 | TMS+常规西药 | SXT+常规西药 | GGS+常规西药 | YXDM+常规西药 |
|---|---|---|---|---|---|
| TMS+常规西药 | 0.54(0.08~3.54) | ||||
| SXT+常规西药 | 0.77(0.17~3.39) | 1.41(0.29~6.77) | |||
| GGS+常规西药 | 0.62(0.09~4.09) | 1.14(0.16~8.03) | 0.81(0.17~3.94) | ||
| YXDM+常规西药 | 0.27(0.04~1.66) | 0.50(0.08~3.26) | 0.35(0.08~1.57) | 0.44(0.07~2.87) | |
| 常规西药 | 2.20(0.62~7.87) | 4.04(1.03~15.90)a | 2.86(1.34~6.14)a | 3.53(0.88~14.09) | 8.09(2.25~29.12)a |
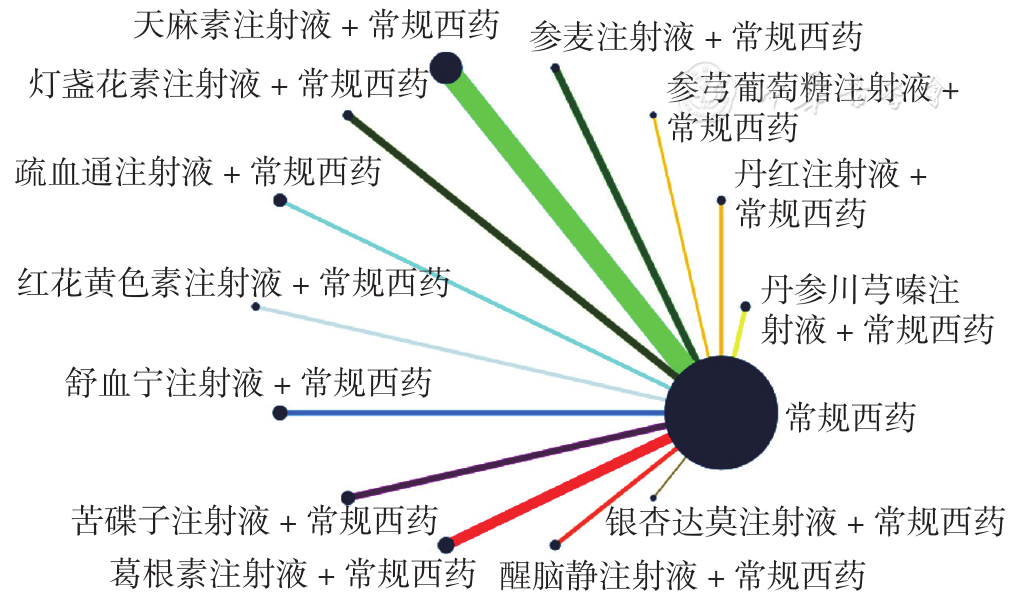
Figure 5 Network relationship diagram of left vertebral artery blood flow velocity in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | DSCXQ+常规西药 | DH+常规西药 | SXPTT+常规西药 | SM+常规西药 | TMS+常规西药 | DZHS+常规西药 | SXT+常规西药 |
|---|---|---|---|---|---|---|---|
| DH+常规西药 | 1.78(-4.44~8.00) | ||||||
| SXPTT+常规西药 | -1.11(-8.84~6.63) | -2.89(-10.64~4.86) | |||||
| SM+常规西药 | 0.45(-6.00~6.91) | -1.33(-7.80~5.15) | 1.56(-6.38~9.50) | ||||
| TMS+常规西药 | -0.09(-4.95~4.78) | -1.87(-6.76~3.02) | 1.02(-5.69~7.73) | -0.54(-5.72~4.64) | |||
| DZHS+常规西药 | -5.84(-12.33~0.65) | -7.62(-14.13~-1.11)a | -4.73(-12.70~3.24) | -6.29(-13.03~0.44) | -5.75(-10.98~-0.52)a | ||
| SXT+常规西药 | 0.99(-5.17~7.15) | -0.79(-6.97~5.39) | 2.09(-5.61~9.80) | 0.53(-5.88~6.95) | 1.07(-3.74~5.89) | 6.83(0.37~13.28)a | |
| HHHSS+常规西药 | -2.47(-8.70~3.76) | -4.25(-10.50~2.00) | -1.36(-9.12~6.39) | -2.92(-9.41~3.56) | -2.38(-7.29~2.52) | 3.37(-3.15~9.89) | -3.46(-9.65~2.74) |
| SXN+常规西药 | -0.43(-6.08~5.21) | -2.22(-7.89~3.46) | 0.67(-6.63~7.97) | -0.89(-6.82~5.04) | -0.35(-4.49~3.79) | 5.40(-0.56~11.37) | -1.42(-7.03~4.18) |
| KDZ+常规西药 | -1.25(-6.92~4.42) | -3.03(-8.72~2.67) | -0.14(-7.46~7.17) | -1.70(-7.65~4.25) | -1.16(-5.33~3.01) | 4.59(-1.40~10.58) | -2.24(-7.87~3.39) |
| GGS+常规西药 | -0.43(-5.84~4.97) | -2.21(-7.64~3.22) | 0.67(-6.44~7.79) | -0.89(-6.58~4.81) | -0.35(-4.15~3.46) | 5.41(-0.33~11.15) | -1.42(-6.78~3.94) |
| XNJ+常规西药 | -7.49(-13.71~-1.26)a | -9.27(-15.51~-3.02)a | -6.38(-14.14~1.37) | -7.94(-14.42~-1.46)a | -7.40(-12.30~-2.50)a | -1.65(-8.18~4.88) | -8.47(-14.66~-2.29)a |
| YXDM+常规西药 | 1.14(-6.39~8.68) | -0.64(-8.19~6.92) | 2.25(-6.59~11.09) | 0.69(-7.06~8.44) | 1.23(-5.25~7.72) | 6.98(-0.80~14.76) | 0.16(-7.35~7.66) |
| 常规西药 | 5.15(0.77~9.54)a | 3.37(-1.04~7.79) | 6.26(-0.11~12.63) | 4.70(-0.04~9.44) | 5.24(3.13~7.35)a | 10.99(6.20~15.78)a | 4.17(-0.16~8.50) |
| 干预措施 | HHHSS+常规西药 | SXN+常规西药 | KDZ+常规西药 | GGS+常规西药 | XNJ+常规西药 | YXDM+常规西药 | |
| DH+常规西药 | |||||||
| SXPTT+常规西药 | |||||||
| SM+常规西药 | |||||||
| TMS+常规西药 | |||||||
| DZHS+常规西药 | |||||||
| SXT+常规西药 | |||||||
| HHHSS+常规西药 | |||||||
| SXN+常规西药 | 2.03(-3.65~7.72) | ||||||
| KDZ+常规西药 | 1.22(-4.48~6.93) | -0.81(-5.88~4.25) | |||||
| GGS+常规西药 | 2.04(-3.41~7.48) | 0.00(-4.76~4.77) | 0.82(-3.98~5.61) | ||||
| XNJ+常规西药 | -5.02(-11.27~1.24)a | -7.05(-12.73~-1.37)a | -6.24(-11.94~-0.54)a | -7.05(-12.49~-1.62)a | |||
| YXDM+常规西药 | 3.61(-3.95~11.18) | 1.58(-5.51~8.67) | 2.39(-4.72~9.50) | 1.58(-5.32~8.48) | 8.63(1.07~16.19)a | ||
| 常规西药 | 7.62(3.20~12.05)a | 5.59(2.03~9.15)a | 6.40(2.80~10.00)a | 5.59(2.42~8.75)a | 12.64(8.22~17.06)a | 4.01(-2.12~10.14) |
Table 4 Network meta-analysis of left vertebral artery blood flow velocity in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | DSCXQ+常规西药 | DH+常规西药 | SXPTT+常规西药 | SM+常规西药 | TMS+常规西药 | DZHS+常规西药 | SXT+常规西药 |
|---|---|---|---|---|---|---|---|
| DH+常规西药 | 1.78(-4.44~8.00) | ||||||
| SXPTT+常规西药 | -1.11(-8.84~6.63) | -2.89(-10.64~4.86) | |||||
| SM+常规西药 | 0.45(-6.00~6.91) | -1.33(-7.80~5.15) | 1.56(-6.38~9.50) | ||||
| TMS+常规西药 | -0.09(-4.95~4.78) | -1.87(-6.76~3.02) | 1.02(-5.69~7.73) | -0.54(-5.72~4.64) | |||
| DZHS+常规西药 | -5.84(-12.33~0.65) | -7.62(-14.13~-1.11)a | -4.73(-12.70~3.24) | -6.29(-13.03~0.44) | -5.75(-10.98~-0.52)a | ||
| SXT+常规西药 | 0.99(-5.17~7.15) | -0.79(-6.97~5.39) | 2.09(-5.61~9.80) | 0.53(-5.88~6.95) | 1.07(-3.74~5.89) | 6.83(0.37~13.28)a | |
| HHHSS+常规西药 | -2.47(-8.70~3.76) | -4.25(-10.50~2.00) | -1.36(-9.12~6.39) | -2.92(-9.41~3.56) | -2.38(-7.29~2.52) | 3.37(-3.15~9.89) | -3.46(-9.65~2.74) |
| SXN+常规西药 | -0.43(-6.08~5.21) | -2.22(-7.89~3.46) | 0.67(-6.63~7.97) | -0.89(-6.82~5.04) | -0.35(-4.49~3.79) | 5.40(-0.56~11.37) | -1.42(-7.03~4.18) |
| KDZ+常规西药 | -1.25(-6.92~4.42) | -3.03(-8.72~2.67) | -0.14(-7.46~7.17) | -1.70(-7.65~4.25) | -1.16(-5.33~3.01) | 4.59(-1.40~10.58) | -2.24(-7.87~3.39) |
| GGS+常规西药 | -0.43(-5.84~4.97) | -2.21(-7.64~3.22) | 0.67(-6.44~7.79) | -0.89(-6.58~4.81) | -0.35(-4.15~3.46) | 5.41(-0.33~11.15) | -1.42(-6.78~3.94) |
| XNJ+常规西药 | -7.49(-13.71~-1.26)a | -9.27(-15.51~-3.02)a | -6.38(-14.14~1.37) | -7.94(-14.42~-1.46)a | -7.40(-12.30~-2.50)a | -1.65(-8.18~4.88) | -8.47(-14.66~-2.29)a |
| YXDM+常规西药 | 1.14(-6.39~8.68) | -0.64(-8.19~6.92) | 2.25(-6.59~11.09) | 0.69(-7.06~8.44) | 1.23(-5.25~7.72) | 6.98(-0.80~14.76) | 0.16(-7.35~7.66) |
| 常规西药 | 5.15(0.77~9.54)a | 3.37(-1.04~7.79) | 6.26(-0.11~12.63) | 4.70(-0.04~9.44) | 5.24(3.13~7.35)a | 10.99(6.20~15.78)a | 4.17(-0.16~8.50) |
| 干预措施 | HHHSS+常规西药 | SXN+常规西药 | KDZ+常规西药 | GGS+常规西药 | XNJ+常规西药 | YXDM+常规西药 | |
| DH+常规西药 | |||||||
| SXPTT+常规西药 | |||||||
| SM+常规西药 | |||||||
| TMS+常规西药 | |||||||
| DZHS+常规西药 | |||||||
| SXT+常规西药 | |||||||
| HHHSS+常规西药 | |||||||
| SXN+常规西药 | 2.03(-3.65~7.72) | ||||||
| KDZ+常规西药 | 1.22(-4.48~6.93) | -0.81(-5.88~4.25) | |||||
| GGS+常规西药 | 2.04(-3.41~7.48) | 0.00(-4.76~4.77) | 0.82(-3.98~5.61) | ||||
| XNJ+常规西药 | -5.02(-11.27~1.24)a | -7.05(-12.73~-1.37)a | -6.24(-11.94~-0.54)a | -7.05(-12.49~-1.62)a | |||
| YXDM+常规西药 | 3.61(-3.95~11.18) | 1.58(-5.51~8.67) | 2.39(-4.72~9.50) | 1.58(-5.32~8.48) | 8.63(1.07~16.19)a | ||
| 常规西药 | 7.62(3.20~12.05)a | 5.59(2.03~9.15)a | 6.40(2.80~10.00)a | 5.59(2.42~8.75)a | 12.64(8.22~17.06)a | 4.01(-2.12~10.14) |
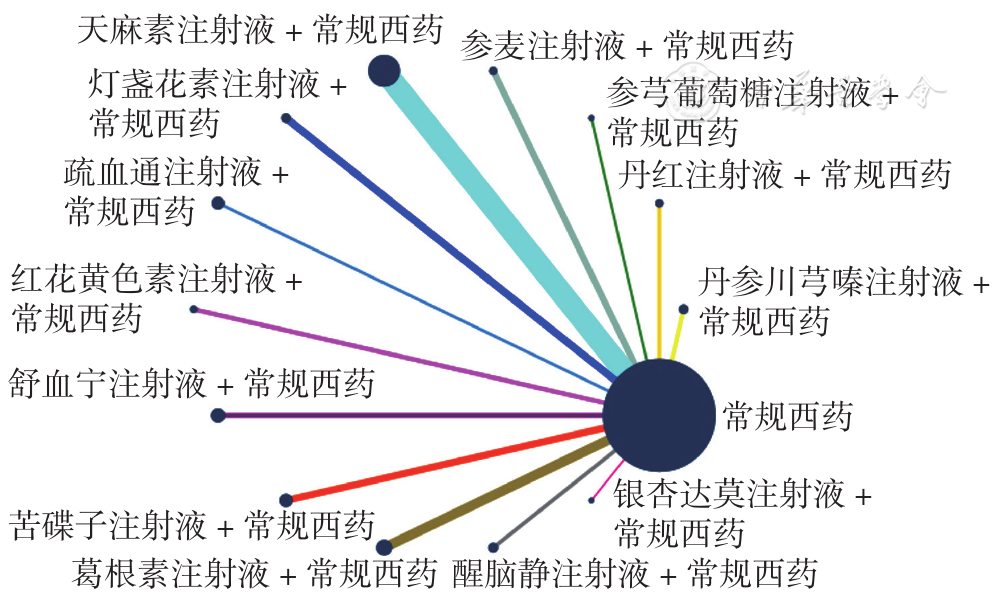
Figure 6 Network relationship diagram of right vertebral artery blood flow velocity in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | DSCXQ+常规西药 | DH+常规西药 | SXPTT+常规西药 | SM+常规西药 | TMS+常规西药 | DZHS+常规西药 | SXT+常规西药 |
|---|---|---|---|---|---|---|---|
| DH+常规西药 | 0.20(-7.33~7.73) | ||||||
| SXPTT+常规西药 | -1.10(-10.39~8.19) | -1.31(-10.62~8.01) | |||||
| SM+常规西药 | -0.43(-8.12~7.25) | -0.64(-8.35~7.08) | 0.67(-8.77~10.11) | ||||
| TMS+常规西药 | -1.31(-7.18~4.57) | -1.51(-7.43~4.41) | -0.20(-8.24~7.84) | -0.87(-6.99~5.24) | |||
| DZHS+常规西药 | -6.63(-14.37~1.11) | -6.83(-14.60~0.94) | -5.53(-15.01~3.96) | -6.20(-14.11~1.72) | -5.32(-11.50~0.85) | ||
| SXT+常规西药 | 1.13(-6.32~8.59) | 0.93(-6.55~8.42) | 2.24(-7.02~11.49) | 1.57(-6.07~9.21) | 2.44(-3.38~8.26) | 7.76(0.07~15.46)a | |
| HHHSS+常规西药 | -5.21(-12.71~2.30) | -5.41(-12.95~2.13) | -4.11(-13.40~5.19) | -4.78(-12.47~2.91) | -3.90(-9.79~1.98) | 1.42(-6.32~9.17) | -6.34(-13.80~1.12) |
| SXN+常规西药 | -1.35(-8.18~5.47) | -1.55(-8.42~5.31) | -0.25(-9.01~8.51) | -0.92(-7.95~6.11) | -0.04(-5.04~4.95) | 5.28(-1.81~12.37) | -2.49(-9.26~4.29) |
| KDZ+常规西药 | -0.32(-7.19~6.54) | -0.53(-7.43~6.38) | 0.78(-8.01~9.57) | 0.11(-6.96~7.18) | 0.98(-4.06~6.03) | 6.31(-0.82~13.43) | -1.46(-8.27~5.36) |
| GGS+常规西药 | -0.71(-7.24~5.81) | -0.92(-7.48~5.64) | 0.39(-8.14~8.91) | -0.28(-7.02~6.46) | 0.59(-3.98~5.16) | 5.92(-0.88~12.71) | -1.85(-8.32~4.62) |
| XNJ+常规西药 | -12.51(-20.02~-5.00)a | -12.71(-20.25~-5.17)a | -11.40(-20.71~-2.10)a | -12.07(-19.77~-4.38)a | -11.20(-17.09~-5.31)a | -5.88(-13.63~1.88) | -13.64(-21.11~-6.18)a |
| YXDM+常规西药 | 0.63(-8.49~9.75) | 0.42(-8.72~9.57) | 1.73(-8.91~12.37) | 1.06(-8.21~10.33) | 1.93(-5.91~9.78) | 7.26(-2.06~16.57) | -0.51(-9.59~8.58) |
| 常规西药 | 3.97(-1.33~9.27) | 3.76(-1.58~9.11) | 5.07(-2.56~12.70) | 4.40(-1.16~9.96) | 5.27(2.74~7.81)a | 10.60(4.96~16.23)a | 2.83(-2.40~8.07) |
| 干预措施 | HHHSS+常规西药 | SXN+常规西药 | KDZ+常规西药 | GGS+常规西药 | XNJ+常规西药 | YXDM+常规西药 | |
| DH+常规西药 | |||||||
| SXPTT+常规西药 | |||||||
| SM+常规西药 | |||||||
| TMS+常规西药 | |||||||
| DZHS+常规西药 | |||||||
| SXT+常规西药 | |||||||
| HHHSS+常规西药 | |||||||
| SXN+常规西药 | 3.86(-2.98~10.69) | ||||||
| KDZ+常规西药 | 4.89(-1.99~11.76) | 1.03(-5.10~7.15) | |||||
| GGS+常规西药 | 4.49(-2.04~11.03) | 0.64(-5.10~6.38) | -0.39(-6.18~5.39) | ||||
| XNJ+常规西药 | -7.30(-14.82~0.22) | -11.15(-18.00~-4.31)a | -12.18(-19.06~-5.30)a | -11.79(-18.33~-5.25)a | |||
| YXDM+常规西药 | 5.84(-3.29~14.96) | 1.98(-6.60~10.56) | 0.95(-7.66~9.56) | 1.34(-7.00~9.68) | 13.13(4.00~22.26)a | ||
| 常规西药 | 9.18(3.86~14.49)a | 5.32(1.02~9.62)a | 4.29(-0.07~8.65) | 4.68(0.88~8.48)a | 16.47(11.15~21.79)a | 3.34(-4.08~10.76) |
Table 5 Network meta-analysis of left vertebral artery blood flow velocity in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | DSCXQ+常规西药 | DH+常规西药 | SXPTT+常规西药 | SM+常规西药 | TMS+常规西药 | DZHS+常规西药 | SXT+常规西药 |
|---|---|---|---|---|---|---|---|
| DH+常规西药 | 0.20(-7.33~7.73) | ||||||
| SXPTT+常规西药 | -1.10(-10.39~8.19) | -1.31(-10.62~8.01) | |||||
| SM+常规西药 | -0.43(-8.12~7.25) | -0.64(-8.35~7.08) | 0.67(-8.77~10.11) | ||||
| TMS+常规西药 | -1.31(-7.18~4.57) | -1.51(-7.43~4.41) | -0.20(-8.24~7.84) | -0.87(-6.99~5.24) | |||
| DZHS+常规西药 | -6.63(-14.37~1.11) | -6.83(-14.60~0.94) | -5.53(-15.01~3.96) | -6.20(-14.11~1.72) | -5.32(-11.50~0.85) | ||
| SXT+常规西药 | 1.13(-6.32~8.59) | 0.93(-6.55~8.42) | 2.24(-7.02~11.49) | 1.57(-6.07~9.21) | 2.44(-3.38~8.26) | 7.76(0.07~15.46)a | |
| HHHSS+常规西药 | -5.21(-12.71~2.30) | -5.41(-12.95~2.13) | -4.11(-13.40~5.19) | -4.78(-12.47~2.91) | -3.90(-9.79~1.98) | 1.42(-6.32~9.17) | -6.34(-13.80~1.12) |
| SXN+常规西药 | -1.35(-8.18~5.47) | -1.55(-8.42~5.31) | -0.25(-9.01~8.51) | -0.92(-7.95~6.11) | -0.04(-5.04~4.95) | 5.28(-1.81~12.37) | -2.49(-9.26~4.29) |
| KDZ+常规西药 | -0.32(-7.19~6.54) | -0.53(-7.43~6.38) | 0.78(-8.01~9.57) | 0.11(-6.96~7.18) | 0.98(-4.06~6.03) | 6.31(-0.82~13.43) | -1.46(-8.27~5.36) |
| GGS+常规西药 | -0.71(-7.24~5.81) | -0.92(-7.48~5.64) | 0.39(-8.14~8.91) | -0.28(-7.02~6.46) | 0.59(-3.98~5.16) | 5.92(-0.88~12.71) | -1.85(-8.32~4.62) |
| XNJ+常规西药 | -12.51(-20.02~-5.00)a | -12.71(-20.25~-5.17)a | -11.40(-20.71~-2.10)a | -12.07(-19.77~-4.38)a | -11.20(-17.09~-5.31)a | -5.88(-13.63~1.88) | -13.64(-21.11~-6.18)a |
| YXDM+常规西药 | 0.63(-8.49~9.75) | 0.42(-8.72~9.57) | 1.73(-8.91~12.37) | 1.06(-8.21~10.33) | 1.93(-5.91~9.78) | 7.26(-2.06~16.57) | -0.51(-9.59~8.58) |
| 常规西药 | 3.97(-1.33~9.27) | 3.76(-1.58~9.11) | 5.07(-2.56~12.70) | 4.40(-1.16~9.96) | 5.27(2.74~7.81)a | 10.60(4.96~16.23)a | 2.83(-2.40~8.07) |
| 干预措施 | HHHSS+常规西药 | SXN+常规西药 | KDZ+常规西药 | GGS+常规西药 | XNJ+常规西药 | YXDM+常规西药 | |
| DH+常规西药 | |||||||
| SXPTT+常规西药 | |||||||
| SM+常规西药 | |||||||
| TMS+常规西药 | |||||||
| DZHS+常规西药 | |||||||
| SXT+常规西药 | |||||||
| HHHSS+常规西药 | |||||||
| SXN+常规西药 | 3.86(-2.98~10.69) | ||||||
| KDZ+常规西药 | 4.89(-1.99~11.76) | 1.03(-5.10~7.15) | |||||
| GGS+常规西药 | 4.49(-2.04~11.03) | 0.64(-5.10~6.38) | -0.39(-6.18~5.39) | ||||
| XNJ+常规西药 | -7.30(-14.82~0.22) | -11.15(-18.00~-4.31)a | -12.18(-19.06~-5.30)a | -11.79(-18.33~-5.25)a | |||
| YXDM+常规西药 | 5.84(-3.29~14.96) | 1.98(-6.60~10.56) | 0.95(-7.66~9.56) | 1.34(-7.00~9.68) | 13.13(4.00~22.26)a | ||
| 常规西药 | 9.18(3.86~14.49)a | 5.32(1.02~9.62)a | 4.29(-0.07~8.65) | 4.68(0.88~8.48)a | 16.47(11.15~21.79)a | 3.34(-4.08~10.76) |
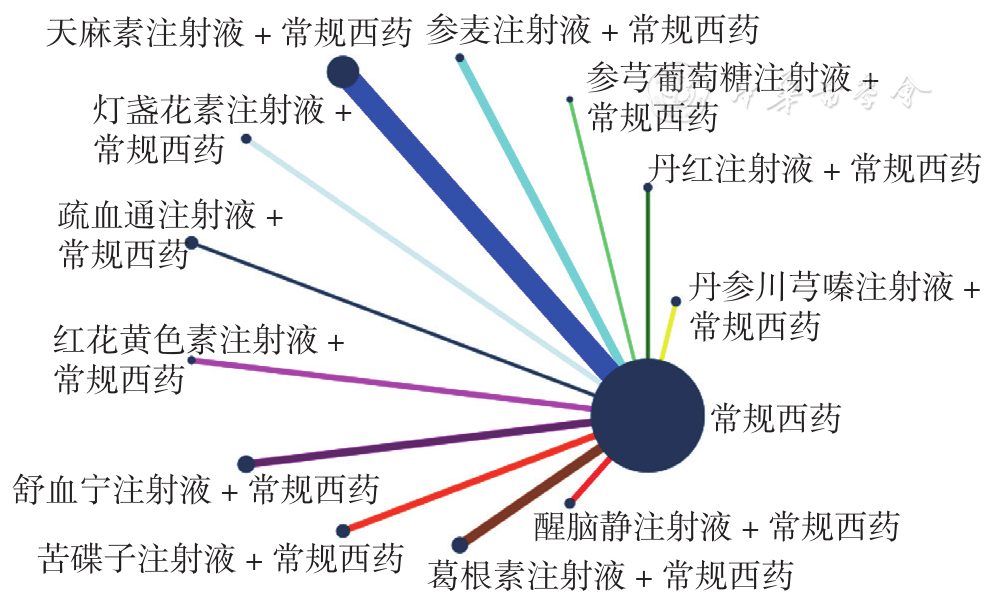
Figure 7 Network relationship diagram of basal artery blood flow velocity in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | DSCXQ+常规西药 | DH+常规西药 | SXPTT+常规西药 | SM+常规西药 | TMS+常规西药 | DZHS+常规西药 |
|---|---|---|---|---|---|---|
| DH+常规西药 | 0.65(-2.17~3.48) | |||||
| SXPTT+常规西药 | -1.28(-5.25~2.68) | -1.94(-5.86~1.98) | ||||
| SM+常规西药 | -0.39(-3.91~3.12) | -1.05(-4.52~2.42) | 0.89(-3.56~5.34) | |||
| TMS+常规西药 | 0.28(-2.00~2.56) | -0.37(-2.58~1.83) | 1.56(-1.98~5.11) | 0.67(-2.37~3.71) | ||
| DZHS+常规西药 | -4.77(-7.98~-1.55)a | -5.42(-8.58~-2.25)a | -3.48(-7.69~0.73) | -4.37(-8.17~-0.58)a | -5.05(-7.73~-2.36)a | |
| SXT+常规西药 | -0.10(-2.90~2.70) | -0.75(-3.50~1.99) | 1.18(-2.72~5.09) | 0.29(-3.16~3.74) | -0.38(-2.55~1.79) | 4.67(1.52~7.81)a |
| HHHSS+常规西药 | -2.88(-5.89~0.14) | -3.53(-6.49~-0.57)a | -1.59(-5.66~2.47) | -2.48(-6.11~1.15) | -3.16(-5.60~-0.71)a | 1.89(-1.45~5.23) |
| SXN+常规西药 | -0.08(-2.55~2.39) | -0.73(-3.15~1.68) | 1.20(-2.48~4.89) | 0.31(-2.88~3.51) | -0.36(-2.11~1.39) | 4.69(1.83~7.54)a |
| KDZ+常规西药 | 0.77(-1.87~3.41) | 0.12(-2.47~2.70) | 2.05(-1.74~5.85) | 1.16(-2.16~4.49) | 0.49(-1.48~2.46) | 5.54(2.53~8.54)a |
| GGS+常规西药 | -0.57(-3.11~1.98) | -1.22(-3.70~1.26) | 0.72(-3.01~4.44) | -0.17(-3.42~3.07) | -0.84(-2.67~0.98) | 4.20(1.28~7.12)a |
| XNJ+常规西药 | -2.37(-5.40~0.67) | -3.02(-6.00~-0.04)a | -1.08(-5.16~2.99) | -1.97(-5.61~1.67) | -2.65(-5.11~-0.18)a | 2.40(-0.96~5.75) |
| 常规西药 | 4.55(2.51~6.58)a | 3.89(1.94~5.85)a | 5.83(2.43~9.23)a | 4.94(2.07~7.81)a | 4.27(3.25~5.28)a | 9.31(6.82~11.80)a |
| 干预措施 | SXT+常规西药 | HHHSS+常规西药 | SXN+常规西药 | KDZ+常规西药 | GGS+常规西药 | XNJ+常规西药 |
| DH+常规西药 | ||||||
| SXPTT+常规西药 | ||||||
| SM+常规西药 | ||||||
| TMS+常规西药 | ||||||
| DZHS+常规西药 | ||||||
| SXT+常规西药 | ||||||
| HHHSS+常规西药 | -2.78(-5.72~0.17) | |||||
| SXN+常规西药 | 0.02(-2.37~2.41) | 2.80(0.16~5.44)a | ||||
| KDZ+常规西药 | 0.87(-1.69~3.43) | 3.65(0.85~6.44)a | 0.85(-1.35~3.05) | |||
| GGS+常规西药 | -0.47(-2.92~1.99) | 2.31(-0.39~5.01) | -0.49(-2.57~1.60) | -1.33(-3.60~0.94) | ||
| XNJ+常规西药 | -2.27(-5.22~0.69) | 0.51(-2.65~3.67) | -2.29(-4.96~0.39) | -3.14(-5.94~-0.33)a | -1.80(-4.51~0.91) | |
| 常规西药 | 4.65(2.73~6.57)a | 7.42(5.20~9.65)a | 4.63(3.21~6.04)a | 3.78(2.09~5.46)a | 5.11(3.59~6.63)a | 6.91(4.67~9.16)a |
Table 6 Network meta analysis of basal arterial blood flow velocity in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | DSCXQ+常规西药 | DH+常规西药 | SXPTT+常规西药 | SM+常规西药 | TMS+常规西药 | DZHS+常规西药 |
|---|---|---|---|---|---|---|
| DH+常规西药 | 0.65(-2.17~3.48) | |||||
| SXPTT+常规西药 | -1.28(-5.25~2.68) | -1.94(-5.86~1.98) | ||||
| SM+常规西药 | -0.39(-3.91~3.12) | -1.05(-4.52~2.42) | 0.89(-3.56~5.34) | |||
| TMS+常规西药 | 0.28(-2.00~2.56) | -0.37(-2.58~1.83) | 1.56(-1.98~5.11) | 0.67(-2.37~3.71) | ||
| DZHS+常规西药 | -4.77(-7.98~-1.55)a | -5.42(-8.58~-2.25)a | -3.48(-7.69~0.73) | -4.37(-8.17~-0.58)a | -5.05(-7.73~-2.36)a | |
| SXT+常规西药 | -0.10(-2.90~2.70) | -0.75(-3.50~1.99) | 1.18(-2.72~5.09) | 0.29(-3.16~3.74) | -0.38(-2.55~1.79) | 4.67(1.52~7.81)a |
| HHHSS+常规西药 | -2.88(-5.89~0.14) | -3.53(-6.49~-0.57)a | -1.59(-5.66~2.47) | -2.48(-6.11~1.15) | -3.16(-5.60~-0.71)a | 1.89(-1.45~5.23) |
| SXN+常规西药 | -0.08(-2.55~2.39) | -0.73(-3.15~1.68) | 1.20(-2.48~4.89) | 0.31(-2.88~3.51) | -0.36(-2.11~1.39) | 4.69(1.83~7.54)a |
| KDZ+常规西药 | 0.77(-1.87~3.41) | 0.12(-2.47~2.70) | 2.05(-1.74~5.85) | 1.16(-2.16~4.49) | 0.49(-1.48~2.46) | 5.54(2.53~8.54)a |
| GGS+常规西药 | -0.57(-3.11~1.98) | -1.22(-3.70~1.26) | 0.72(-3.01~4.44) | -0.17(-3.42~3.07) | -0.84(-2.67~0.98) | 4.20(1.28~7.12)a |
| XNJ+常规西药 | -2.37(-5.40~0.67) | -3.02(-6.00~-0.04)a | -1.08(-5.16~2.99) | -1.97(-5.61~1.67) | -2.65(-5.11~-0.18)a | 2.40(-0.96~5.75) |
| 常规西药 | 4.55(2.51~6.58)a | 3.89(1.94~5.85)a | 5.83(2.43~9.23)a | 4.94(2.07~7.81)a | 4.27(3.25~5.28)a | 9.31(6.82~11.80)a |
| 干预措施 | SXT+常规西药 | HHHSS+常规西药 | SXN+常规西药 | KDZ+常规西药 | GGS+常规西药 | XNJ+常规西药 |
| DH+常规西药 | ||||||
| SXPTT+常规西药 | ||||||
| SM+常规西药 | ||||||
| TMS+常规西药 | ||||||
| DZHS+常规西药 | ||||||
| SXT+常规西药 | ||||||
| HHHSS+常规西药 | -2.78(-5.72~0.17) | |||||
| SXN+常规西药 | 0.02(-2.37~2.41) | 2.80(0.16~5.44)a | ||||
| KDZ+常规西药 | 0.87(-1.69~3.43) | 3.65(0.85~6.44)a | 0.85(-1.35~3.05) | |||
| GGS+常规西药 | -0.47(-2.92~1.99) | 2.31(-0.39~5.01) | -0.49(-2.57~1.60) | -1.33(-3.60~0.94) | ||
| XNJ+常规西药 | -2.27(-5.22~0.69) | 0.51(-2.65~3.67) | -2.29(-4.96~0.39) | -3.14(-5.94~-0.33)a | -1.80(-4.51~0.91) | |
| 常规西药 | 4.65(2.73~6.57)a | 7.42(5.20~9.65)a | 4.63(3.21~6.04)a | 3.78(2.09~5.46)a | 5.11(3.59~6.63)a | 6.91(4.67~9.16)a |
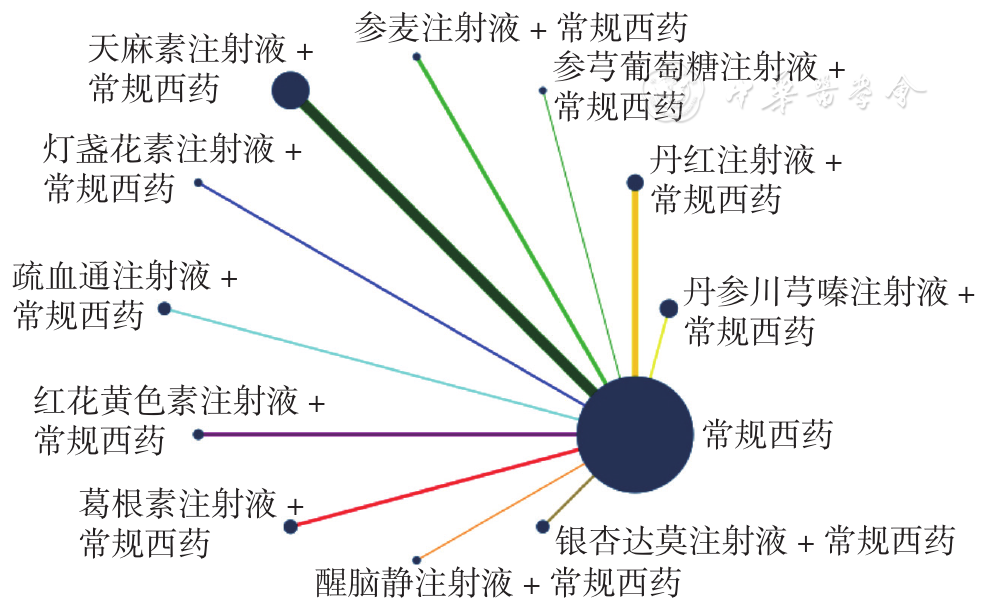
Figure 8 Network relationship diagram of whole blood high shear viscosity in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | DSCXQ+常规西药 | DH+常规西药 | SXPTT+常规西药 | SM+常规西药 | TMS+常规西药 | DZHS+常规西药 |
|---|---|---|---|---|---|---|
| DH+常规西药 | 0.78(0.03~1.53)a | |||||
| SXPTT+常规西药 | -0.10(-1.10~0.90) | -0.88(-1.92~0.16) | ||||
| SM+常规西药 | 1.10(-0.24~2.45) | 0.32(-1.05~1.70) | 1.20(-0.33~2.73) | |||
| TMS+常规西药 | -0.05(-0.65~0.55) | -0.83(-1.50~-0.16)a | 0.05(-0.89~0.99) | -1.15(-2.45~0.15) | ||
| DZHS+常规西药 | 0.59(-0.50~1.69) | -0.19(-1.32~0.95) | 0.69(-0.62~2.00) | -0.51(-2.10~1.08) | 0.64(-0.40~1.68) | |
| SXT+常规西药 | 0.78(-0.27~1.84) | 0.00(-1.09~1.09) | 0.88(-0.40~2.16) | -0.32(-1.88~1.24) | 0.83(-0.16~1.83) | 0.19(-1.16~1.54) |
| HHHSS+常规西药 | 0.73(-0.12~1.57) | -0.06(-0.95~0.84) | 0.82(-0.29~1.94) | -0.38(-1.80~1.05) | 0.78(0.00~1.55)a | 0.13(-1.07~1.33) |
| GGS+常规西药 | 0.19(-0.63~1.02) | -0.59(-1.46~0.28) | 0.29(-0.81~1.38) | -0.91(-2.33~0.50) | 0.24(-0.51~0.99) | -0.40(-1.59~0.78) |
| XNJ+常规西药 | -0.41(-1.42~0.61) | -1.19(-2.25~-0.13)a | -0.31(-1.56~0.94) | -1.51(-3.05~0.03) | -0.36(-1.32~0.60) | -1.00(-2.33~0.33) |
| YXDM+常规西药 | -0.08(-0.88~0.72) | -0.86(-1.71~-0.01)a | 0.02(-1.06~1.09) | -1.18(-2.59~0.22) | -0.03(-0.75~0.69) | -0.67(-1.84~0.49) |
| 常规西药 | -0.94(-1.43~-0.45)a | -1.72(-2.29~-1.15)a | -0.84(-1.71~0.03) | -2.04(-3.29~-0.79)a | -0.89(-1.24~-0.54)a | -1.53(-2.51~-0.55)a |
| 干预措施 | SXT+常规西药 | HHHSS+常规西药 | GGS+常规西药 | XNJ+常规西药 | YXDM+常规西药 | |
| DH+常规西药 | ||||||
| SXPTT+常规西药 | ||||||
| SM+常规西药 | ||||||
| TMS+常规西药 | ||||||
| DZHS+常规西药 | ||||||
| SXT+常规西药 | ||||||
| HHHSS+常规西药 | -0.06(-1.22~1.10) | |||||
| GGS+常规西药 | -0.59(-1.74~0.55) | -0.54(-1.49~0.42) | ||||
| XNJ+常规西药 | -1.19(-2.48~0.10) | -1.13(-2.26~-0.01)a | -0.60(-1.71~0.51) | |||
| YXDM+常规西药 | -0.86(-1.99~0.26) | -0.81(-1.74~0.13) | -0.27(-1.19~0.64) | 0.33(-0.77~1.42) | ||
| 常规西药 | -1.72(-2.65~-0.79)a | -1.66(-2.35~-0.98)a | -1.13(-1.79~-0.47)a | -0.53(-1.42~0.36) | -0.86(-1.49~-0.22)a |
Table 7 Network meta analysis of whole blood high shear viscosity in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | DSCXQ+常规西药 | DH+常规西药 | SXPTT+常规西药 | SM+常规西药 | TMS+常规西药 | DZHS+常规西药 |
|---|---|---|---|---|---|---|
| DH+常规西药 | 0.78(0.03~1.53)a | |||||
| SXPTT+常规西药 | -0.10(-1.10~0.90) | -0.88(-1.92~0.16) | ||||
| SM+常规西药 | 1.10(-0.24~2.45) | 0.32(-1.05~1.70) | 1.20(-0.33~2.73) | |||
| TMS+常规西药 | -0.05(-0.65~0.55) | -0.83(-1.50~-0.16)a | 0.05(-0.89~0.99) | -1.15(-2.45~0.15) | ||
| DZHS+常规西药 | 0.59(-0.50~1.69) | -0.19(-1.32~0.95) | 0.69(-0.62~2.00) | -0.51(-2.10~1.08) | 0.64(-0.40~1.68) | |
| SXT+常规西药 | 0.78(-0.27~1.84) | 0.00(-1.09~1.09) | 0.88(-0.40~2.16) | -0.32(-1.88~1.24) | 0.83(-0.16~1.83) | 0.19(-1.16~1.54) |
| HHHSS+常规西药 | 0.73(-0.12~1.57) | -0.06(-0.95~0.84) | 0.82(-0.29~1.94) | -0.38(-1.80~1.05) | 0.78(0.00~1.55)a | 0.13(-1.07~1.33) |
| GGS+常规西药 | 0.19(-0.63~1.02) | -0.59(-1.46~0.28) | 0.29(-0.81~1.38) | -0.91(-2.33~0.50) | 0.24(-0.51~0.99) | -0.40(-1.59~0.78) |
| XNJ+常规西药 | -0.41(-1.42~0.61) | -1.19(-2.25~-0.13)a | -0.31(-1.56~0.94) | -1.51(-3.05~0.03) | -0.36(-1.32~0.60) | -1.00(-2.33~0.33) |
| YXDM+常规西药 | -0.08(-0.88~0.72) | -0.86(-1.71~-0.01)a | 0.02(-1.06~1.09) | -1.18(-2.59~0.22) | -0.03(-0.75~0.69) | -0.67(-1.84~0.49) |
| 常规西药 | -0.94(-1.43~-0.45)a | -1.72(-2.29~-1.15)a | -0.84(-1.71~0.03) | -2.04(-3.29~-0.79)a | -0.89(-1.24~-0.54)a | -1.53(-2.51~-0.55)a |
| 干预措施 | SXT+常规西药 | HHHSS+常规西药 | GGS+常规西药 | XNJ+常规西药 | YXDM+常规西药 | |
| DH+常规西药 | ||||||
| SXPTT+常规西药 | ||||||
| SM+常规西药 | ||||||
| TMS+常规西药 | ||||||
| DZHS+常规西药 | ||||||
| SXT+常规西药 | ||||||
| HHHSS+常规西药 | -0.06(-1.22~1.10) | |||||
| GGS+常规西药 | -0.59(-1.74~0.55) | -0.54(-1.49~0.42) | ||||
| XNJ+常规西药 | -1.19(-2.48~0.10) | -1.13(-2.26~-0.01)a | -0.60(-1.71~0.51) | |||
| YXDM+常规西药 | -0.86(-1.99~0.26) | -0.81(-1.74~0.13) | -0.27(-1.19~0.64) | 0.33(-0.77~1.42) | ||
| 常规西药 | -1.72(-2.65~-0.79)a | -1.66(-2.35~-0.98)a | -1.13(-1.79~-0.47)a | -0.53(-1.42~0.36) | -0.86(-1.49~-0.22)a |
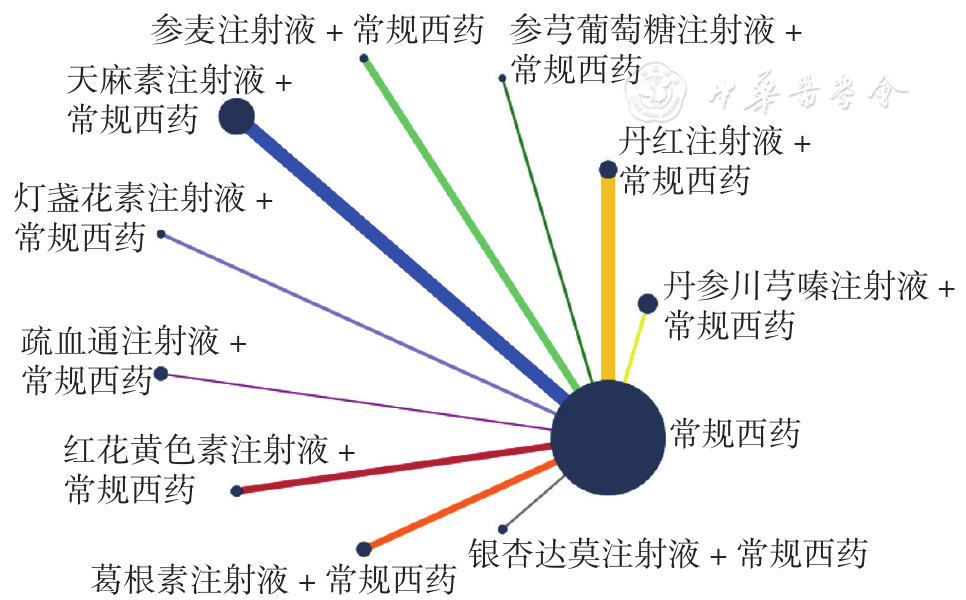
Figure 9 Network relationship diagram of whole blood low shear viscosity in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | DSCXQ+常规西药 | DH+常规西药 | SXPTT+常规西药 | SM+常规西药 | TMS+常规西药 |
|---|---|---|---|---|---|
| DH+常规西药 | 0.34(-0.65~1.34) | ||||
| SXPTT+常规西药 | -0.14(-1.49~1.20) | -0.49(-1.93~0.95) | |||
| SM+常规西药 | 0.92(-1.28~3.11) | 0.57(-1.68~2.83) | 1.06(-1.37~3.49) | ||
| TMS+常规西药 | 0.75(-0.01~1.51) | 0.41(-0.51~1.33) | 0.90(-0.40~2.19) | -0.16(-2.33~2.00) | |
| DZHS+常规西药 | -0.14(-1.65~1.36) | -0.49(-2.08~1.10) | 0.00(-1.83~1.83) | -1.06(-3.58~1.46) | -0.90(-2.36~0.56) |
| SXT+常规西药 | 1.28(0.05~2.50)a | 0.93(-0.40~2.26) | 1.42(-0.19~3.03) | 0.36(-2.01~2.73) | 0.52(-0.65~1.69) |
| HHHSS+常规西药 | 0.26(-0.84~1.37) | -0.08(-1.30~1.14) | 0.41(-1.11~1.93) | -0.65(-2.96~1.66) | -0.49(-1.53~0.55) |
| GGS+常规西药 | 0.38(-0.74~1.50) | 0.04(-1.20~1.27) | 0.52(-1.01~2.06) | -0.54(-2.85~1.78) | -0.37(-1.43~0.68) |
| YXDM+常规西药 | -0.52(-1.85~0.80) | -0.87(-2.29~0.55) | -0.38(-2.07~1.31) | -1.44(-3.86~0.98) | -1.28(-2.55~-0.00)a |
| 常规西药 | -1.63(-2.23~-1.04)a | -1.98(-2.77~-1.19)a | -1.49(-2.69~-0.29)a | -2.55(-4.66~-0.44)a | -2.39(-2.86~-1.92)a |
| 干预措施 | DZHS+常规西药 | SXT+常规西药 | HHHSS+常规西药 | GGS+常规西药 | YXDM+常规西药 |
| DH+常规西药 | |||||
| SXPTT+常规西药 | |||||
| SM+常规西药 | |||||
| TMS+常规西药 | |||||
| DZHS+常规西药 | |||||
| SXT+常规西药 | 1.42(-0.33~3.17) | ||||
| HHHSS+常规西药 | 0.41(-1.25~2.07) | -1.01(-2.43~0.40) | |||
| GGS+常规西药 | 0.52(-1.15~2.20) | -0.90(-2.32~0.53) | 0.12(-1.21~1.44) | ||
| YXDM+常规西药 | -0.38(-2.20~1.44) | -1.80(-3.39~-0.21)a | -0.79(-2.29~0.71) | -0.90(-2.42~0.61) | |
| 常规西药 | -1.49(-2.87~-0.11)a | -2.91(-3.98~-1.84)a | -1.90(-2.83~-0.97)a | -2.01(-2.96~-1.07)a | -1.11(-2.29~0.07)a |
Table 8 Network meta analysis of whole blood low shear viscosity in PCIV patients treated with different traditional Chinese medicine injections in combination
| 干预措施 | DSCXQ+常规西药 | DH+常规西药 | SXPTT+常规西药 | SM+常规西药 | TMS+常规西药 |
|---|---|---|---|---|---|
| DH+常规西药 | 0.34(-0.65~1.34) | ||||
| SXPTT+常规西药 | -0.14(-1.49~1.20) | -0.49(-1.93~0.95) | |||
| SM+常规西药 | 0.92(-1.28~3.11) | 0.57(-1.68~2.83) | 1.06(-1.37~3.49) | ||
| TMS+常规西药 | 0.75(-0.01~1.51) | 0.41(-0.51~1.33) | 0.90(-0.40~2.19) | -0.16(-2.33~2.00) | |
| DZHS+常规西药 | -0.14(-1.65~1.36) | -0.49(-2.08~1.10) | 0.00(-1.83~1.83) | -1.06(-3.58~1.46) | -0.90(-2.36~0.56) |
| SXT+常规西药 | 1.28(0.05~2.50)a | 0.93(-0.40~2.26) | 1.42(-0.19~3.03) | 0.36(-2.01~2.73) | 0.52(-0.65~1.69) |
| HHHSS+常规西药 | 0.26(-0.84~1.37) | -0.08(-1.30~1.14) | 0.41(-1.11~1.93) | -0.65(-2.96~1.66) | -0.49(-1.53~0.55) |
| GGS+常规西药 | 0.38(-0.74~1.50) | 0.04(-1.20~1.27) | 0.52(-1.01~2.06) | -0.54(-2.85~1.78) | -0.37(-1.43~0.68) |
| YXDM+常规西药 | -0.52(-1.85~0.80) | -0.87(-2.29~0.55) | -0.38(-2.07~1.31) | -1.44(-3.86~0.98) | -1.28(-2.55~-0.00)a |
| 常规西药 | -1.63(-2.23~-1.04)a | -1.98(-2.77~-1.19)a | -1.49(-2.69~-0.29)a | -2.55(-4.66~-0.44)a | -2.39(-2.86~-1.92)a |
| 干预措施 | DZHS+常规西药 | SXT+常规西药 | HHHSS+常规西药 | GGS+常规西药 | YXDM+常规西药 |
| DH+常规西药 | |||||
| SXPTT+常规西药 | |||||
| SM+常规西药 | |||||
| TMS+常规西药 | |||||
| DZHS+常规西药 | |||||
| SXT+常规西药 | 1.42(-0.33~3.17) | ||||
| HHHSS+常规西药 | 0.41(-1.25~2.07) | -1.01(-2.43~0.40) | |||
| GGS+常规西药 | 0.52(-1.15~2.20) | -0.90(-2.32~0.53) | 0.12(-1.21~1.44) | ||
| YXDM+常规西药 | -0.38(-2.20~1.44) | -1.80(-3.39~-0.21)a | -0.79(-2.29~0.71) | -0.90(-2.42~0.61) | |
| 常规西药 | -1.49(-2.87~-0.11)a | -2.91(-3.98~-1.84)a | -1.90(-2.83~-0.97)a | -2.01(-2.96~-1.07)a | -1.11(-2.29~0.07)a |
| [1] |
|
| [2] |
|
| [3] |
王烁. 椎—基底动脉供血不足性眩晕的文献分析及临床证候研究[D].北京:北京中医药大学,2012.
|
| [4] |
卓实,江川,陈文玲,等. 中西药联合治疗椎基底动脉供血不足性眩晕疗效的Meta分析及中药处方用药规律分析[J]. 临床合理用药,2023,16(31):20-24. DOI:10.15887/j.cnki.13-1389/r.2023.31.006.
|
| [5] |
|
| [6] |
《中成药治疗优势病种临床应用指南》标准化项目组. 中成药治疗眩晕相关疾病临床应用指南(2022年)[J]. 中国中西医结合杂志,2023,43(10):1157-1166. DOI:10.7661/j.cjim.20230906.154.
|
| [7] |
中华医学会神经病学分会,中华神经科杂志编辑委员会. 眩晕诊治专家共识[J]. 中华神经科杂志,2010,43(5):369-374. DOI:10.3760/cma.j.issn.1006-7876.2010.05.016.
|
| [8] | |
| [9] |
吕传真,周良辅. 实用神经病学[M]. 4版. 上海:上海科学技术出版社,2014.
|
| [10] |
中华人民共和国卫生部.中药新药临床研究指导原则(第一辑)[M]. 北京:人民卫生出版社,1993:24-25.
|
| [11] |
常留军,岳慧丽,李文战,等. 丹参川芎嗪注射液联合尼麦角林治疗后循环缺血性眩晕的临床效果 [J]. 慢性病学杂志,2020,21(1):144-145,148. DOI:10.16440/j.cnki.1674-8166.2020.01.053.
|
| [12] |
姚灵枝. 丹参川芎嗪注射液联合盐酸氟桂利嗪胶囊治疗后循环缺血性头晕的临床效果[J]. 中国民康医学,2018,30(16):26-27. DOI:10.3969/j.issn.1672-0369.2018.16.013.
|
| [13] |
王飒,赵淑宪,华锋,等. 盐酸倍他司汀注射液联合丹参川芎嗪注射液治疗后循环缺血性眩晕的临床效果[J]. 临床医学研究与实践,2022,7(4):125-128. DOI:10.19347/j.cnki.2096-1413.202204034.
|
| [14] |
张祖康,周风彩. 丹红注射液对椎基底动脉供血不足眩晕患者血流动力学及中医症候积分的影响 [J]. 反射疗法与康复医学,2022,3(9):69-72. DOI:10.19347/j.cnki.2096-1413.202204034.
|
| [15] |
冯子凌. 丹红注射液治疗椎-基底动脉供血不足疗效观察[J]. 中西医结合心脑血管病杂志,2013,11(8):961-962. DOI:10.3969/j.issn.1672-1349.2013.08.037.
|
| [16] |
徐波. 丹红注射液联合氟桂利嗪治疗椎基底动脉供血不足眩晕的疗效观察[J]. 现代药物与临床,2017,32(9):1651-1654. DOI:10.7501/j.issn.1674-5515.2017.09.011.
|
| [17] |
王春玲,田娟,张琴. 丹红注射液联合盐酸丁咯地尔治疗椎-基底动脉供血不足患者临床疗效观察[J]. 浙江临床医学,2010,12(5):486-487. DOI:10.3969/j.issn.1008-7664.2010.05.018.
|
| [18] |
张勇,李媛. 参芎葡萄糖注射液治疗慢阻肺稳定期合并椎-基底动脉供血不足性眩晕疗效观察[J]. 陕西中医,2013,34(12):1623-1625. DOI:10.3969/j.issn.1000-7369.2013.12.028.
|
| [19] |
邱红. 参芎葡萄糖注射液联合前列地尔治疗后循环缺血性眩晕的临床观察[J]. 医学信息,2013,26(28):99-100.
|
| [20] |
马良. 参麦注射液联合倍他司汀注射液治疗后循环缺血性眩晕的临床疗效分析[J]. 中西医结合研究,2017,9(3):140-141. DOI:10.3969/j.issn.1674-4616.2017.03.009.
|
| [21] |
肖展翅,吕衍文,宛丰,等. 参麦注射液联合倍他司汀注射液治疗后循环缺血眩晕的临床研究 [J]. 中西医结合心脑血管病杂志,2014,12(12):1453-1455. DOI:10.3969/j.issn.16721349.2014.12.008.
|
| [22] |
邢海辉,李本红,李巧云,等. 参麦注射液联合甲磺酸倍他司汀片治疗后循环缺血眩晕的临床疗效探讨[J]. 当代医学,2017,23(27):113-115. DOI:10.3969/j.issn.1009-4393.2017.27.050.
|
| [23] |
刘俊花,姜晓蕊. 天麻素注射液联合倍他司汀治疗椎-基底动脉供血不足性眩晕症患者的效果[J]. 中国民康医学,2024,36(6):14-16. DOI:10.3969/j.issn.1672-0369.2024.06.005.
|
| [24] |
宋晓丽. 天麻素治疗椎-基底动脉供血不足性眩晕症的疗效分析[J]. 现代实用医学,2020,32(4):528-529. DOI:10.3969/j.issn.1671-0800.2020.04.057.
|
| [25] | |
| [26] |
相铁辉,王海建,路路. 天麻素注射液联合长春西汀治疗后循环缺血性眩晕的临床效果[J]. 河南医学研究,2020,29(12):2233-2234. DOI:10.3969/j.issn.1004-437X.2020.12.060.
|
| [27] |
韦正新. 天麻素注射液联合长春西汀治疗椎-基底动脉供血不足性眩晕随机平行对照研究 [J]. 实用中医内科杂志,2016,30(7):65-67. DOI:10.13729/j.issn.1671-7813.2016.07.27.
|
| [28] |
吴迪. 天麻素注射液联合马来酸桂哌齐特注射液治疗后循环缺血性眩晕的临床疗效观察[J]. 自我保健,2020(15):139-140.
|
| [29] |
方军涛,张岚,邢珊珊,等.天麻素联合倍他司汀治疗后循环缺血性眩晕临床效果分析[J].中文科技期刊数据库(全文版)医药卫生,2021(5):21-22.
|
| [30] |
叶斌. 天麻素联合倍他司汀治疗后循环缺血性眩晕临床观察[J]. 实用中医药杂志,2023,39(5):951-953.
|
| [31] |
陈洁.天麻素联合倍他司汀治疗后循环缺血性眩晕临床观察[J].中国科技期刊数据库医药,2023(10):55-58.
|
| [32] | |
| [33] |
张岚. 天麻素联合尼麦角林治疗后循环缺血性眩晕临床疗效观察 [J]. 养生保健指南,2019(33):53,94.
|
| [34] |
唐铁钰,张新江,段作伟,等. 天麻素联合甲磺酸倍他司汀治疗后循环缺血性眩晕的疗效及机制研究[J]. 中药材,2017,40(11):2706-2709. DOI:10.13863/j.issn1001-4454.2017.11.047.
|
| [35] |
陶海军,徐先勇,胡官印. 天麻素联合西药治疗椎基底动脉供血不足性眩晕症临床观察[J]. 光明中医,2023,38(17):3429-3432. DOI:10.3969/j.issn.1003-8914.2023.17.045.
|
| [36] |
曾利,史忠,高全杰,等. 法舒地尔联合天麻素治疗椎基底动脉供血不足性眩晕的疗效观察[J]. 西部医学,2012,24(12):2395-2396. DOI:10.3969/j.issn.1672-3511.2012.12.059.
|
| [37] |
张春驰,董艳娟,贺昕,等. 前列地尔联合灯盏花素治疗后循环缺血眩晕的疗效观察 [J]. 疑难病杂志,2011,10(9):688-689.
|
| [38] |
郑泽荣,刘晖,宋小珍. 灯盏花素注射液治疗椎-基底动脉供血不足58例[J]. 中西医结合心脑血管病杂志,2006,4(6):484-486. DOI:10.3969/j.issn.1672-1349.2006.06.009.
|
| [39] |
王美华. 倍他司汀联合疏血通注射液治疗椎-基底动脉供血不足性眩晕症的临床观察[J]. 深圳中西医结合杂志,2017,27(15):38-40. DOI:10.16458/j.cnki.1007-0893.2017.15.016.
|
| [40] |
侯胜利. 氟桂利嗪胶囊联合疏血通注射液治疗椎-基底动脉供血不足性眩晕的疗效分析[J]. 天津药学,2018,30(1):48-50. DOI:10.3969/j.issn.1006-5687.2018.01.017.
|
| [41] |
郑素平,高赵英. 红花黄色素注射液治疗椎-基底动脉供血不足性眩晕症的临床疗效[J]. 临床合理用药杂志,2019,12(36):81-83. DOI:10.15887/j.cnki.13-1389/r.2019.36.040.
|
| [42] |
李超. 红花黄色素注射液辅助氟桂利嗪治疗椎-基底动脉供血不足性眩晕的效果[J]. 包头医学,2022,46(3):25-27. DOI:10.3969/j.issn.1007-3507.2022.03.012.
|
| [43] |
赵耀. 低分子肝素钙结合舒血宁治疗椎-基底动脉缺血引起眩晕的临床效果分析[J]. 中国医药导刊,2011,13(11):1900-1901. DOI:10.3969/j.issn.1009-0959.2011.11.035.
|
| [44] |
周彩琴. 低分子肝素钠结合舒血宁治疗后循环缺血性眩晕的临床疗效分析[J]. 临床医药文献电子杂志,2018,5(A3):54,61. DOI:10.3877/j.issn.2095-8242.2018.A3.038.
|
| [45] |
谭永峰,班玉霞. 舒血宁注射液联合尼麦角林治疗基底动脉供血不足性眩晕的效果及对脑血灌注量的影响[J]. 临床医学研究与实践,2022,7(29):71-74. DOI:10.19347/j.cnki.2096-1413.202229020.
|
| [46] |
杨牧,程相杰,韩娟,等. 舒血宁注射液联合甲磺酸倍他司汀片治疗后循环缺血眩晕的临床价值研究[J]. 北方药学,2019,16(3):42-43. DOI:10.3969/j.issn.1672-8351.2019.03.029.
|
| [47] |
任钦,戎立辉. 苦碟子注射液联合前列地尔治疗后循环缺血性眩晕的临床观察[J]. 中国药房,2015(21):2931,2933. DOI:10.6039/j.issn.1001-0408.2015.21.19.
|
| [48] |
孙金柱,于晓娜,胡勇. 苦碟子注射液联合纳洛酮治疗后循环缺血性眩晕临床研究[J]. 中国药业,2019,28(17):67-69. DOI:10.3969/j.issn.1006-4931.2019.17.020.
|
| [49] |
李嘉辉. 苦碟子注射液联合纳洛酮治疗后循环缺血性眩晕的临床研究[J]. 现代药物与临床,2018,33(4):745-749. DOI:10.7501/j.issn.1674-5515.2018.04.006.
|
| [50] |
王红洲,王万华,毛慧慧. 尼莫地平联合葛根素治疗后循环缺血性眩晕临床观察[J]. 中西医结合心脑血管病杂志,2011,9(7):804-805. DOI:10.3969/j.issn.1672-1349.2011.07.020.
|
| [51] |
简军. 葛根素注射液治疗椎-基底动脉供血不足性眩晕40例临床观察[J]. 河南中医,2005,25(5):69-70. DOI:10.3969/j.issn.1003-5028.2005.05.058.
|
| [52] |
杨明华,樊素娟. 葛根素注射液治疗椎-基底动脉供血不足性眩晕的疗效观察[J]. 中国医院用药评价与分析,2017,17(5):653-655. DOI:10.14009/j.issn.1672-2124.2017.05.029.
|
| [53] |
张方. 葛根素注射液联合氟桂利嗪治疗椎-基底动脉供血不足性眩晕患者的效果[J]. 河南医学研究,2020,29(6):1073-1074. DOI:10.3969/j.issn.1004-437X.2020.06.050.
|
| [54] |
赵娜,李东旭,王庆海,等. 醒脑静注射液治疗后循环缺血性眩晕的临床疗效观察[J]. 养生保健指南,2019(33):50.
|
| [55] |
田丽丽. 醒脑静注射液联合倍他司汀治疗后循环缺血性眩晕的疗效及对CGRP、ET-1的影响研究[J]. 中国现代药物应用,2023,17(7):113-116. DOI:10.14164/j.cnki.cn11-5581/r.2023.07.034.
|
| [56] |
岳婷,奚耀,王琴,等. 醒脑静注射液联合阿司匹林治疗脑部后循环缺血43例临床观察[J]. 中国民族民间医药,2014,20(23):37-38. DOI:10.3969/j.issn.1007-8517.2014.23.zgmzmjyyzz201423023.
|
| [57] |
温泽云,何祥英,王吾勇,等. 银杏达莫注射液联合倍他司汀治疗后循环缺血眩晕对患者血液黏度的影响[J]. 中国药业,2021,30(15):105-108. DOI:10.3969/j.issn.1006-4931.2021.15.030.
|
| [58] |
李法强,纪艾玲. 银杏达莫注射液联合前列地尔治疗后循环缺血性眩晕临床观察[J]. 湖北中医药大学学报,2015,35(6):25-27. DOI:10.3969/j.issn.1008-987x.2015.06.09.
|
| [59] |
栾琴. 银杏达莫注射液联合前列地尔治疗后循环缺血性眩晕的临床疗效观察及其安全性评价[J]. 国际医药卫生导报,2016,22(10):1432-1435. DOI:10.3760/cma.j.issn.1007-1245.2016.10.032.
|
| [60] |
朱毅,邓大一,黄庆松.后循环缺血单发性眩晕中医药研究进展[J].贵州中医药大学学报,2022,44(5):65-71. DOI:10.16588/j.cnki.issn2096-8426.2022.05.015.
|
| [61] |
刘淑芬,刘淑清. 疏血通注射液对急性脑梗死患者血脂、凝血功能及血液流变学的影响[J]. 中西医结合心脑血管病杂志,2012,10(7):831-833. DOI:10.3969/j.issn.1672-1349.2012.07.033.
|
| [62] |
赵佳源,王小玲,王小芳,等. 疏血通注射液治疗进展性卒中有效性的Meta分析及GRADE证据质量评价[J]. 中国中药杂志,2022,47(3):807-818. DOI:10.19540/j.cnki.cjcmm.20210702.502.
|
| [1] | QUAN Jialin, ZHU Lin, SU Yu, CHEN Zekai, CHEN Ziqi, ZHANG Zhuofan. Research on the Improvement Effect of Exercise Modes on the Executive Function of Overweight or Obese Children or Adolescents: a Network Meta-analysis [J]. Chinese General Practice, 2025, 28(27): 3422-3431. |
| [2] | LI Hao, LI Jiangtao, LIU Dan, WANG Jianjun. Efficacy and Safety of Belimumab, Anifrolumab, and Telitacicept on the Treatment of Systemic Lupus Erythematosus: a Network Meta-analysis [J]. Chinese General Practice, 2025, 28(23): 2924-2933. |
| [3] | MA Panpan, WANG Sijing, YOU Na, DING Dafa, LU Yibing. Efficacy and Safety of Danuglipron and Orforglipron in the Treatment of Type 2 Diabetes Mellitus: a Meta-analysis [J]. Chinese General Practice, 2025, 28(21): 2679-2685. |
| [4] | LIU Caiping, ZHANG Yanhua, TANG Jianpin, WANG Chengpeng, XUE Fengfeng, WANG Huijuan, LI Chuanwei, ZHANG Guangya, LI Huafang. Efficacy and Safety of Long-acting Risperidone Microspheres in the Maintenance Treatment of Schizophrenia [J]. Chinese General Practice, 2025, 28(13): 1622-1627. |
| [5] | CHI Xun, LIU Sisi, CHEN Qiao, HU Yue, WANG Weixian. The Suitability of Four Nutritional Screening Tools for Nutritional Screening in Patients with Cirrhosis: a Network Meta-analysis [J]. Chinese General Practice, 2025, 28(11): 1395-1402. |
| [6] | GU Mingyu, QIN Tingting, QIAO Kun, BAI Xinyuan, WANG Yao, YANG Yutong, LI Xingming. A Network Meta-analysis of Primary Hypertension Management Patterns in China [J]. Chinese General Practice, 2025, 28(10): 1265-1272. |
| [7] | MA Huping, REN Rong, HOU Mei, YUAN Aiyun. Clinical Observation of the New Antiepileptic Drug Perampanel in the Treatment of Refractory Epilepsy in Children Aged 0-18 Years [J]. Chinese General Practice, 2025, 28(02): 250-256. |
| [8] | HU Zhen, LIU Mei, GONG Limei, WANG Jianhong. Application of Vestibular Function Combined with Audiological Examination in Vertigo Diseases [J]. Chinese General Practice, 2024, 27(35): 4435-4438. |
| [9] | GUO Jia, CAO Chunmei, LIU Guochun, ZHENG Man, ZHU Ruihan, LONG Wei. Effects of Different Exercise Types on Sleep in Insomnia Patients: a Network Meta-analysis [J]. Chinese General Practice, 2024, 27(35): 4376-4387. |
| [10] | ZHANG Jiateng, KONG Ming, CHEN Yu, DUAN Zhongping. Study on the Efficacy and Safety of Kuhuang Injection and Kuhuang Granules in the Sequential Treatment of Drug-induced Liver Injury: a Non-inferiority Randomized Controlled Trial [J]. Chinese General Practice, 2024, 27(26): 3249-3254. |
| [11] | YANG Can, LI Ning, LI Xuefei, ZHAO Li, XU Hao, SHI Qi, WANG Yongjun, LIANG Qianqian. Efficacy of Zang Bi Formula in Treating Arthritis and Its Pulmonary Complications in Rheumatoid Arthritis Interstitial Lung Disease Mice [J]. Chinese General Practice, 2024, 27(24): 3015-3022. |
| [12] | HUANG Tengjia, CAO Xi, CHEN Lei, LI Ziying, QIN Lihua. The Effectiveness of Non-pharmacological Treatment for Post-stroke Shoulder-hand Syndrome: a Network Meta-analysis [J]. Chinese General Practice, 2024, 27(23): 2921-2930. |
| [13] | WANG Ting, WANG Haiyan, FU Wenjun. Effect of Chronic Atrophic Gastritis Treated with Different Acupuncture and Moxibustion Therapies: a Network Meta-analysis [J]. Chinese General Practice, 2024, 27(23): 2913-2920. |
| [14] | TAN Shufa, ZHANG Leichang, GAO Qiangqiang, OU Yan, HUANG Shuilan. Efficacy and Safety of Biologics and Small Molecule Drugs in the Treatment of Ulcerative Colitis: a Network Meta-analysis [J]. Chinese General Practice, 2024, 27(17): 2155-2166. |
| [15] | PANG Lan, LI Peifan, ZHU Xiaogang, YANG Zaihong, ZHENG Lei. Deep Transcranial Magnetic Stimulation Combined with Escitalopram Oxalate in the Treatment of Depression: a Randomized Controlled Trial [J]. Chinese General Practice, 2024, 27(17): 2098-2103. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||